Chiral Analysis of Molecules with Multiple Chiral Centers





- Slides: 16

Chiral Analysis of Molecules with Multiple Chiral Centers using Chiral Tag Rotational Spectroscopy Luca Evangelisti Dipartmento di Chimica “Giacomo Ciamician”, Universita Di Bologna ISMS 2019 MH 10 Reilly E. Sonstrom, Kevin J. Mayer, Channing T. West and Brooks H. Pate

Challenges for Quantitative Chiral Analysis 1) Often requires extensive method development to achieve separation by chromatography. 2) Chromatography requires identification of eluting peaks. 3) Analysis within a complex mixture can be difficult. Key Point: Isomer analysis is difficult for mass spectrometry. NMR also has challenges for enantiomer and regioisomer analysis especially for minor components in a sample. 4) Analysis times can be long compared to time scales needed for production monitoring. 5) Additional analysis challenges come from the presence of regioisomers.

Applications of Rotational Spectroscopy in Analytical Chemistry Quantitative Chiral Analysis of Molecules with Multiple Chiral Centers Determine the sample composition for all 2 N stereoisomers Diastereomers: Enantiomers: 2 N-1 distinct molecular structures Non-superimposable mirror images of each diastereomer Enantiomer: Absolute Configuration and Enantiomeric Excess Chiral Tag Rotational Spectroscopy Advantages: 1) No reference samples needed 2) Single instrument for full analysis 3) Potential for high-speed monitoring (Balle-Flygare)

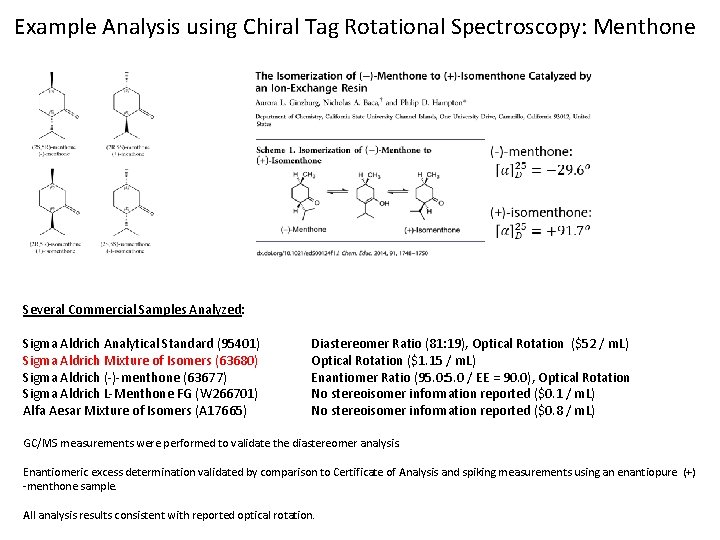
Example Analysis using Chiral Tag Rotational Spectroscopy: Menthone Several Commercial Samples Analyzed: Sigma Aldrich Analytical Standard (95401) Sigma Aldrich Mixture of Isomers (63680) Sigma Aldrich (-)-menthone (63677) Sigma Aldrich L-Menthone FG (W 266701) Alfa Aesar Mixture of Isomers (A 17665) Diastereomer Ratio (81: 19), Optical Rotation ($52 / m. L) Optical Rotation ($1. 15 / m. L) Enantiomer Ratio (95. 0: 5. 0 / EE = 90. 0), Optical Rotation No stereoisomer information reported ($0. 1 / m. L) No stereoisomer information reported ($0. 8 / m. L) GC/MS measurements were performed to validate the diastereomer analysis. Enantiomeric excess determination validated by comparison to Certificate of Analysis and spiking measurements using an enantiopure (+) -menthone sample. All analysis results consistent with reported optical rotation.

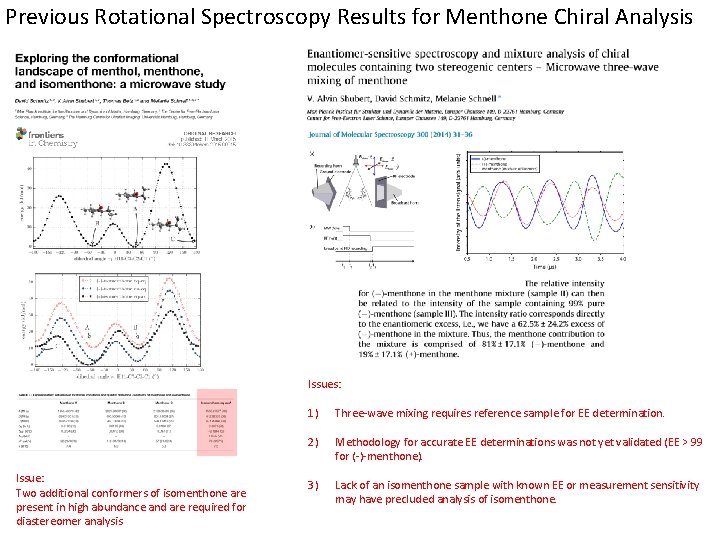
Previous Rotational Spectroscopy Results for Menthone Chiral Analysis Issues: Issue: Two additional conformers of isomenthone are present in high abundance and are required for diastereomer analysis 1) Three-wave mixing requires reference sample for EE determination. 2) Methodology for accurate EE determinations was not yet validated (EE > 99 for (-)-menthone). 3) Lack of an isomenthone sample with known EE or measurement sensitivity may have precluded analysis of isomenthone.

Chiral Tag Analysis by Broadband Rotational Spectroscopy 1) Monomer condition optimization (temperature) Diastereomer analysis (optional) 2) High-Enantiopurity Tag Measurement 0. 1% (S)-butynol in neon EE = 98. 37(9) (WL 02) Double-regulated external reservoir Absolute configuration (13 C substitution) Enantiomeric Excess 3) Racemic Tag Measurement (after purge or clean) Calibration of EE measurement Increased confidence for absolute configuration Low-Frequency Chirped-Pulse Fourier Transform Microwave Spectroscopy (2 -8 GHz)

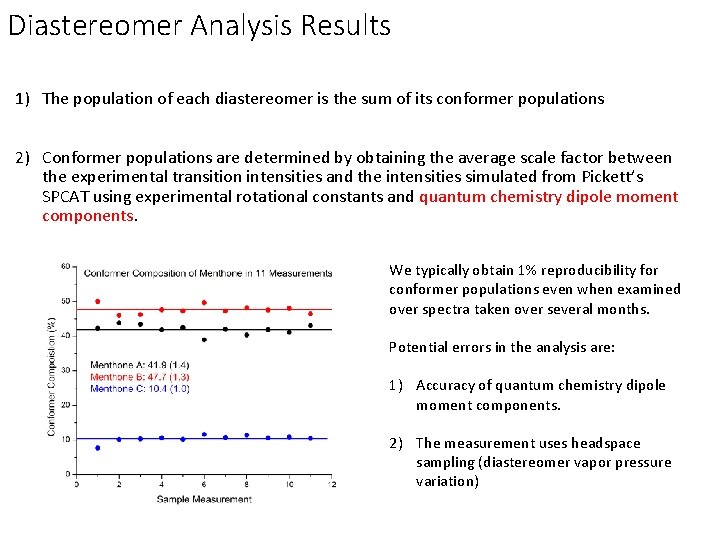
Diastereomer Analysis Results 1) The population of each diastereomer is the sum of its conformer populations 2) Conformer populations are determined by obtaining the average scale factor between the experimental transition intensities and the intensities simulated from Pickett’s SPCAT using experimental rotational constants and quantum chemistry dipole moment components. We typically obtain 1% reproducibility for conformer populations even when examined over spectra taken over several months. Potential errors in the analysis are: 1) Accuracy of quantum chemistry dipole moment components. 2) The measurement uses headspace sampling (diastereomer vapor pressure variation)

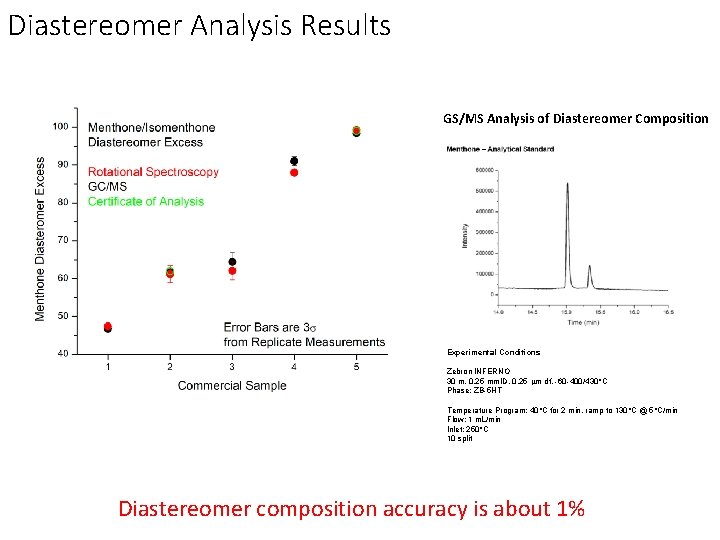
Diastereomer Analysis Results GS/MS Analysis of Diastereomer Composition Experimental Conditions Zebron INFERNO 30 m, 0. 25 mm. ID, 0. 25 m df, -60 -400/430°C Phase: ZB-5 HT Temperature Program: 40°C for 2 min, ramp to 130°C @ 5°C/min Flow: 1 m. L/min Inlet: 250°C 10 split Diastereomer composition accuracy is about 1%

Enantiomer Analysis Enantiomers-to-Diastereomers Strategy using a Chiral Tag Attached using Noncovalent Interactions (2 R, 5 S)-menthone (+)-menthone (2 S, 5 R)-menthone (-)-menthone Rotational Constants A = 1957. 813 MHz B = 695. 020 MHz C = 586. 908 MHz Theory: B 3 LYP D 3 BJ 6 -311++G(d, p) Experiment: A = 1953. 434 MHz B = 694. 516 MHz C = 586. 578 MHz

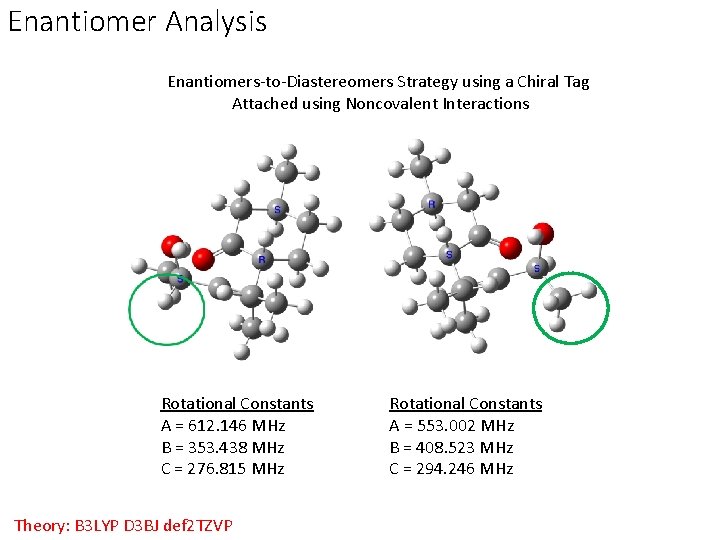
Enantiomer Analysis Enantiomers-to-Diastereomers Strategy using a Chiral Tag Attached using Noncovalent Interactions Rotational Constants A = 612. 146 MHz B = 353. 438 MHz C = 276. 815 MHz Theory: B 3 LYP D 3 BJ def 2 TZVP Rotational Constants A = 553. 002 MHz B = 408. 523 MHz C = 294. 246 MHz

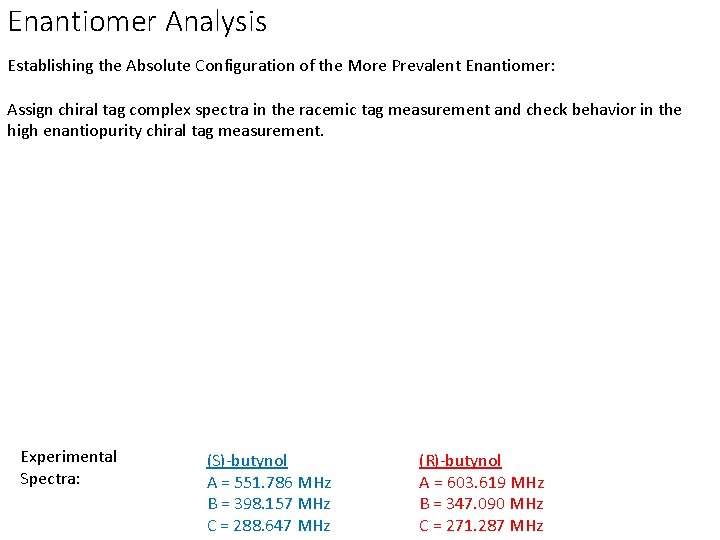
Enantiomer Analysis Establishing the Absolute Configuration of the More Prevalent Enantiomer: Assign chiral tag complex spectra in the racemic tag measurement and check behavior in the high enantiopurity chiral tag measurement. Experimental Spectra: (S)-butynol A = 551. 786 MHz B = 398. 157 MHz C = 288. 647 MHz (R)-butynol A = 603. 619 MHz B = 347. 090 MHz C = 271. 287 MHz

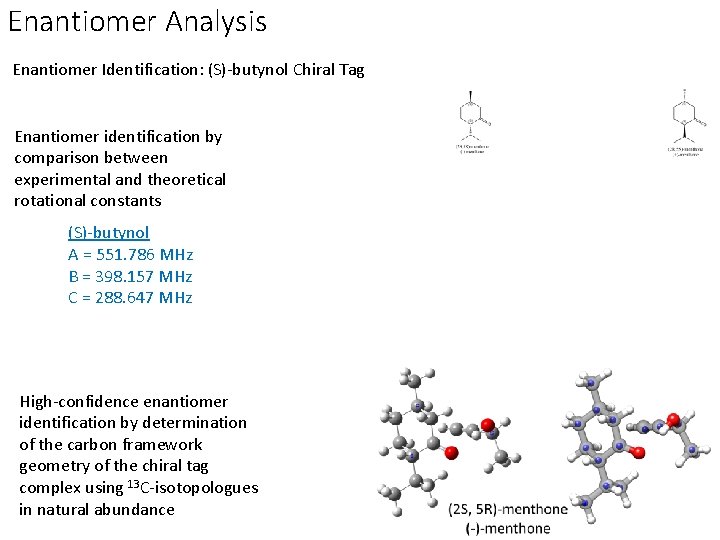
Enantiomer Analysis Enantiomer Identification: (S)-butynol Chiral Tag Enantiomer identification by comparison between experimental and theoretical rotational constants (S)-butynol A = 551. 786 MHz B = 398. 157 MHz C = 288. 647 MHz High-confidence enantiomer identification by determination of the carbon framework geometry of the chiral tag complex using 13 C-isotopologues in natural abundance

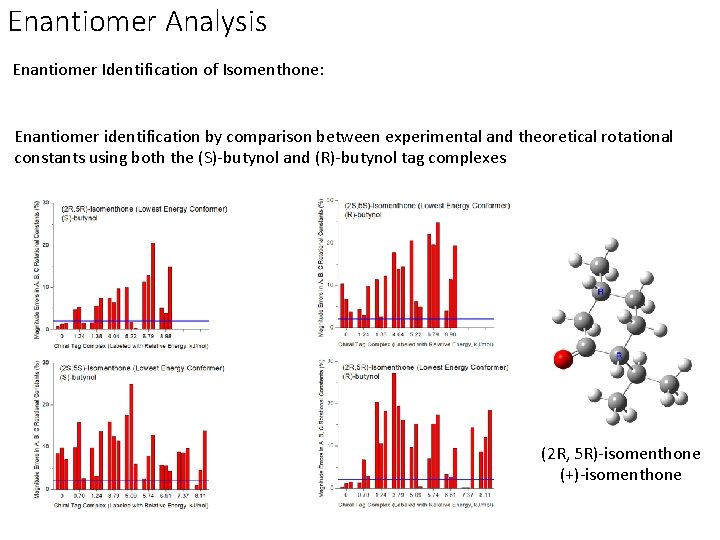
Enantiomer Analysis Enantiomer Identification of Isomenthone: Enantiomer identification by comparison between experimental and theoretical rotational constants using both the (S)-butynol and (R)-butynol tag complexes (2 R, 5 R)-isomenthone (+)-isomenthone

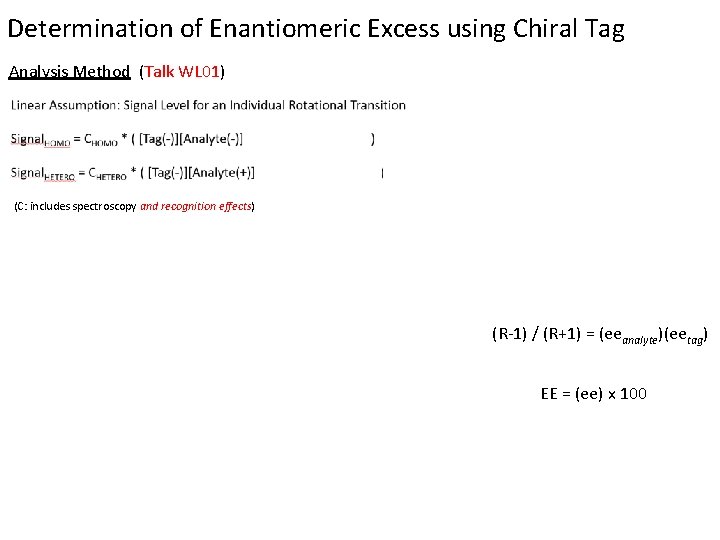
Determination of Enantiomeric Excess using Chiral Tag Analysis Method (Talk WL 01) (C: includes spectroscopy and recognition effects) (R-1) / (R+1) = (eeanalyte)(eetag) EE = (ee) x 100

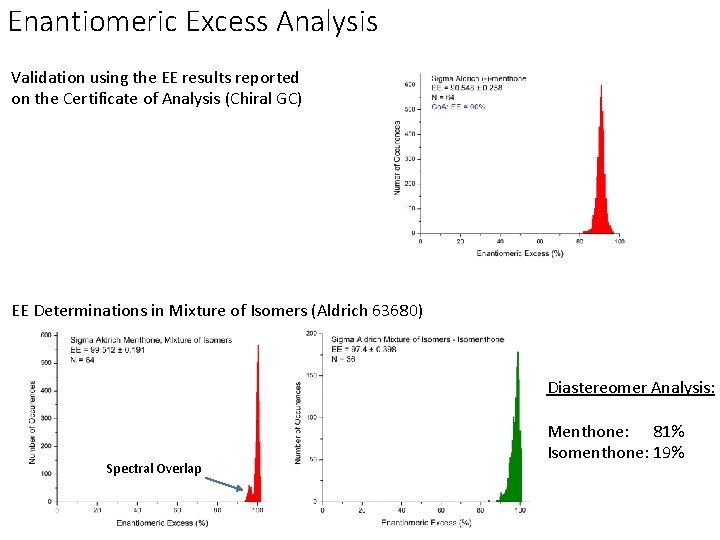
Enantiomeric Excess Analysis Validation using the EE results reported on the Certificate of Analysis (Chiral GC) EE Determinations in Mixture of Isomers (Aldrich 63680) Diastereomer Analysis: Spectral Overlap Menthone: 81% Isomenthone: 19%

Conclusions • Quantitative Chiral Analysis is an important field of analytical chemistry where rotational spectroscopy offers new measurement capabilities • Chiral Tag Analysis has a significant advantage over three-wave mixing that the analysis can be performed without the need of reference samples • The accuracy for both diastereomer and enantiomer analysis is comparable to gas chromatography techniques Acknowledgements: NSF 1531913 Dave Patterson, Walther Caminati, Yunjie Xu, Javix Thomas, David Pratt, Smitty Grubbs, Galen Sedo, Mark Marshall, Helen Leung, Kevin Lehmann, Justin Neill, Frank Marshall, Maria Sanz, Reinhard Doetzer