Childrens Outcomes Research Program The Childrens Hospital Aurora
















- Slides: 26

Children’s Outcomes Research Program The Children’s Hospital Aurora, CO Denver, CO Colorado Health Colorado Outcomes. Health Program Outcomes Program Univ. of Colorado Denver Aurora, CO Willingness to Administer Influenza A (H 1 N 1) 2009 Monovalent Vaccine: A National Physician Survey Sean O’Leary, MD Fellow, Pediatric Infectious Diseases

Background • The appearance of a novel strain of influenza in 2009 led to a global pandemic, the ramifications of which continue to unfold • There was a rapid international response, including the expedited production of a vaccine • Before the vaccine became available, it was unknown how willing primary care physicians would be to administer it

Objectives To determine among U. S. pediatricians (Peds), family medicine physicians (FM), and general internists (GIM): 1) Willingness to administer 2009 H 1 N 1 vaccine with limited information regarding safety and efficacy 2) Barriers to administering the vaccine 3) Preferences about receipt of public healthrelated information about H 1 N 1 disease and vaccine

Context of Survey • 2009 H 1 N 1 virus identified in April 2009 • Development of survey started in early June • Survey administered July through October with content based on early information about anticipated vaccine • Vaccine administration started in October for most of country

Methods: Survey Population Ø Survey conducted in 3 existing sentinel physician networks – Physicians practicing >50% primary care were recruited from random samples of AAP, AAFP and ACP – Quota sampling done to ensure networks similar to overall AAP, AAFP and ACP memberships Ø Previous study* comparing sentinel network methodology to most commonly used method of randomly sampling from AMA masterfile found comparable results with respect to: – Physician and practice characteristics – Responses on surveys regarding vaccine-related issues *Crane LA, Eval & Health Prof, 2008

Methods: Survey Design • Survey developed jointly with CDC with input from national vaccine experts • Pre-tested in advisory committees of Peds, FM, and GIM physicians from across country and piloted among 196 primary care physicians

Survey Administration • Survey Period: July - October 2009 • Administered by Internet or mail depending on preference of physician – 1/3 by mail – 2/3 by Internet

Survey Content • Respondents provided with specific information regarding what was known about H 1 N 1 vaccine at the time • In the context of this information, they were asked about: – Willingness to administer vaccine – Potential barriers to administering

Information Provided • “The FDA has not yet made the decision about whether the new H 1 N 1 vaccine will be a licensed product or a product used under an Emergency Use Authorization (EUA). ” • “If the vaccine is used under EUA it will require that providers give fact sheets to patients and track patient information for the FDA but physicians will not have to obtain informed consent. ” • “The seasonal and H 1 N 1 vaccines will not be combined into one shot but it is likely that seasonal influenza and H 1 N 1 vaccine doses can be given at the same time. ”

Information Provided • “It is anticipated that anyone <50 years will need to receive two doses of the new H 1 N 1 vaccine. ” • “Children <9 years who have not received 2 influenza doses in previous seasons will require 2 doses of seasonal and 2 doses of H 1 N 1 vaccine. ” • “It is not known when H 1 N 1 vaccine will be available but supplies may be extensive. ” • “Side effects of the H 1 N 1 vaccine are expected to be similar to seasonal influenza vaccine. ”

Results: Survey Response • 76% response rate overall Ø FM: 70% (298/424) Ø Peds: 79% (330/416) Ø GIM: 78% (337/432) • Among the specialties, respondents not significantly different from non-respondents with respect to gender, birth year, practice location, region, and practice type

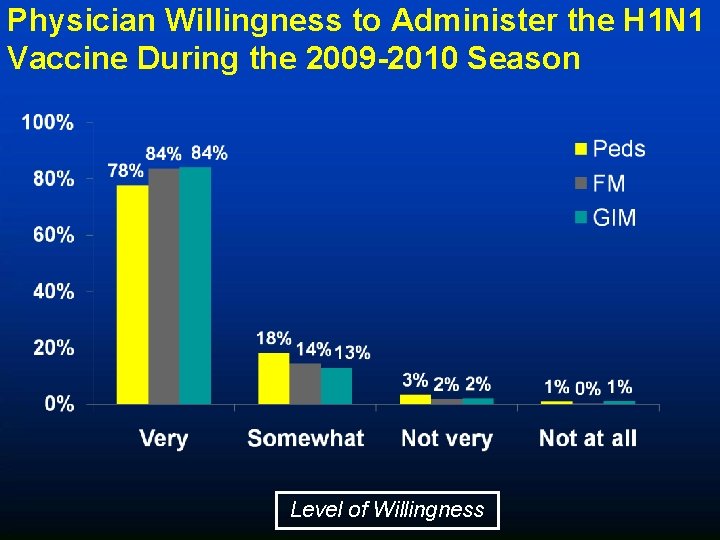
Physician Willingness to Administer the H 1 N 1 Vaccine During the 2009 -2010 Season Level of Willingness

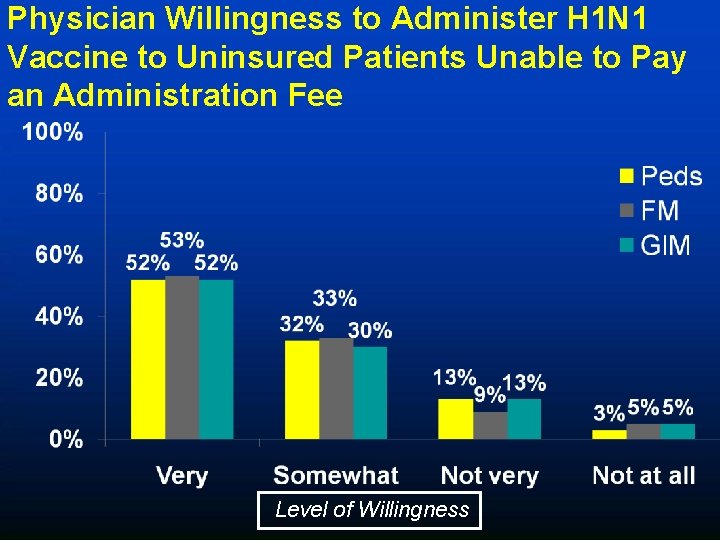
Physician Willingness to Administer H 1 N 1 Vaccine to Uninsured Patients Unable to Pay an Administration Fee Level of Willingness

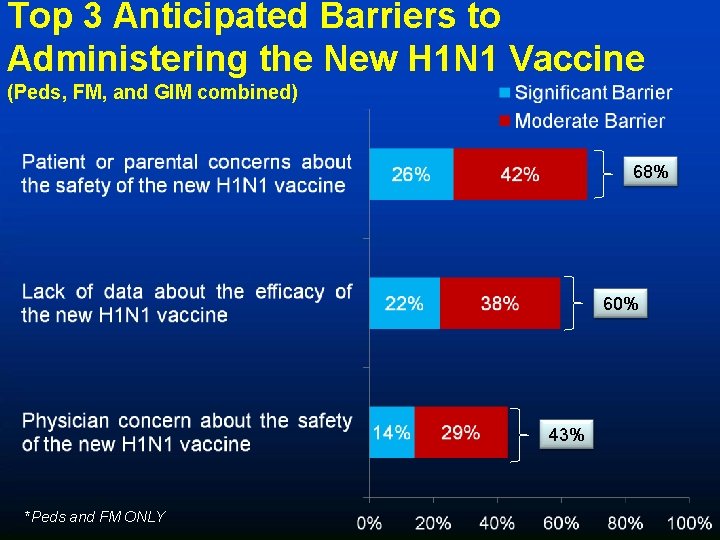
Top 3 Anticipated Barriers to Administering the New H 1 N 1 Vaccine (Peds, FM, and GIM combined) 68% 60% 43% *Peds and FM ONLY

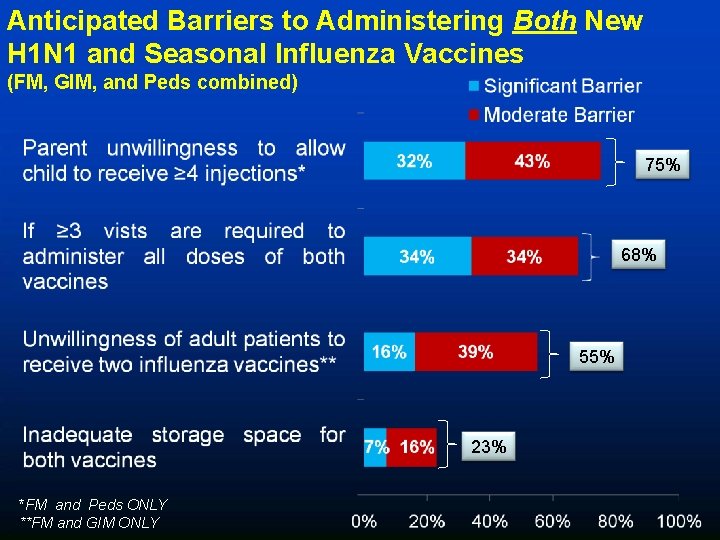
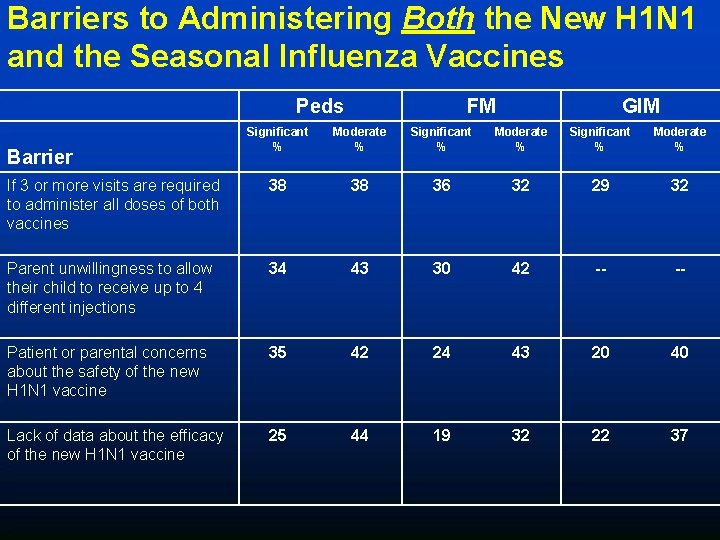
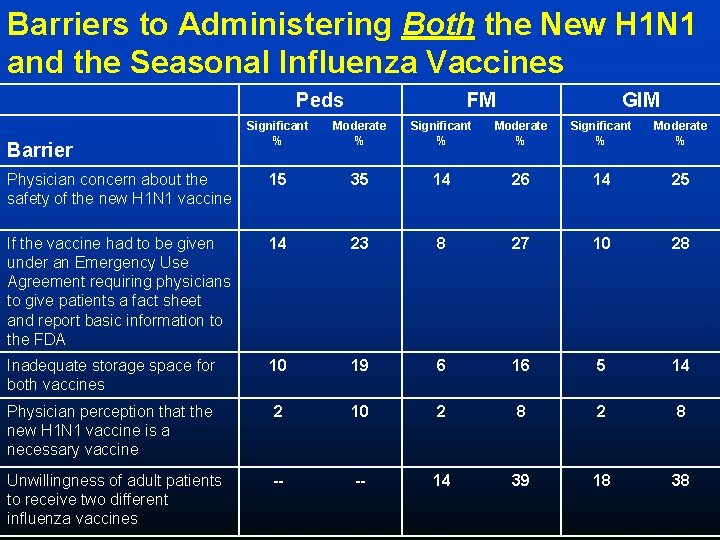
Anticipated Barriers to Administering Both New H 1 N 1 and Seasonal Influenza Vaccines (FM, GIM, and Peds combined) 75% 68% 55% 23% *FM and Peds ONLY **FM and GIM ONLY

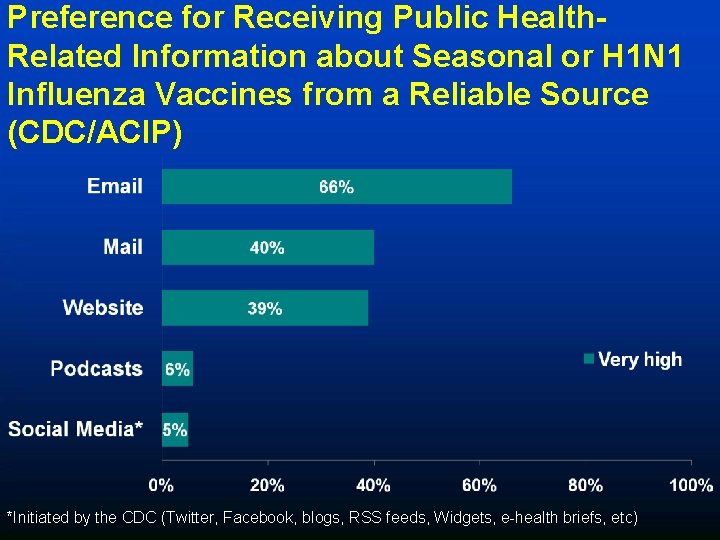
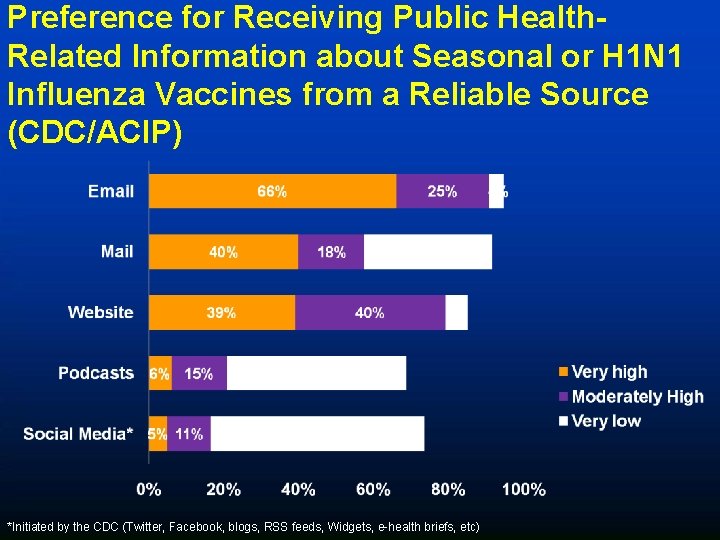
Preference for Receiving Public Health. Related Information about Seasonal or H 1 N 1 Influenza Vaccines from a Reliable Source (CDC/ACIP) *Initiated by the CDC (Twitter, Facebook, blogs, RSS feeds, Widgets, e-health briefs, etc)

Limitations • Respondents may have differed from nonrespondents • Sentinel physicians may differ from physicians overall (prior work suggests not) • Survey results represent reported practice; actual practice not observed • The survey took place during a rapidly changing landscape with respect to disease and vaccine

Summary of Results • The majority of physicians surveyed were very or somewhat willing to provide 2009 H 1 N 1 vaccine • The greatest perceived barriers among physicians were patient and parent safety concerns and the need for 3 or more separate administrations • Email, and updates via the CDC website were the preferred methods of communication

Conclusions • Although potential barriers were noted, our data suggest that private providers would be willing partners in the delivery of a new vaccine in the face of a pandemic, such as the 2009 H 1 N 1 vaccine • Such information will be useful in the future in the event of another pandemic or other emerging infectious disease

Vaccine Policy Collaborative Initiative University of Colorado Denver Principal Investigator - Allison Kempe, MD, MPH • • • Sean O’Leary, MD Matt Daley, MD Lori A. Crane, Ph. D, MPH Laura Hurley, MD, MPH Fran Dong, MS • • • Christine Babbel, MSPH L Miriam Dickinson, Ph. D Christina Suh, MD Laura Seewald, BA Claire Gahm, BA CDC Collaborators • • Shannon Stokley, MPH Pascale Wortley, MD, MPH Funding CDC, through American Academy of Medical Colleges (AAMC)

Additional Information

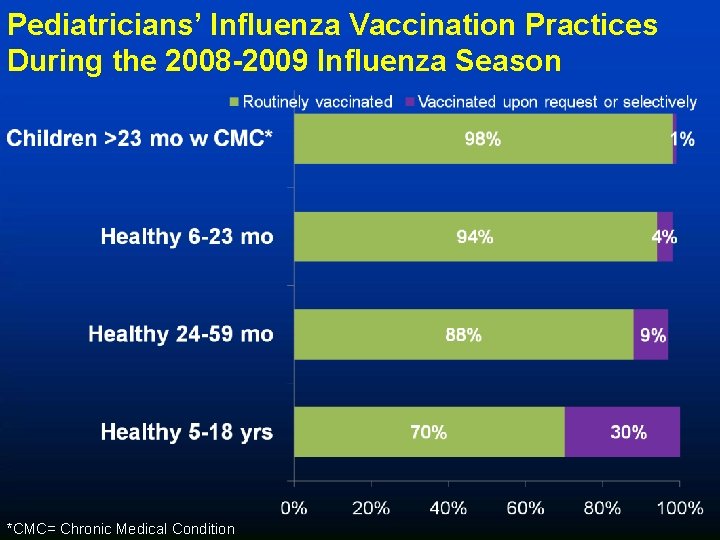
Pediatricians’ Influenza Vaccination Practices During the 2008 -2009 Influenza Season *CMC= Chronic Medical Condition

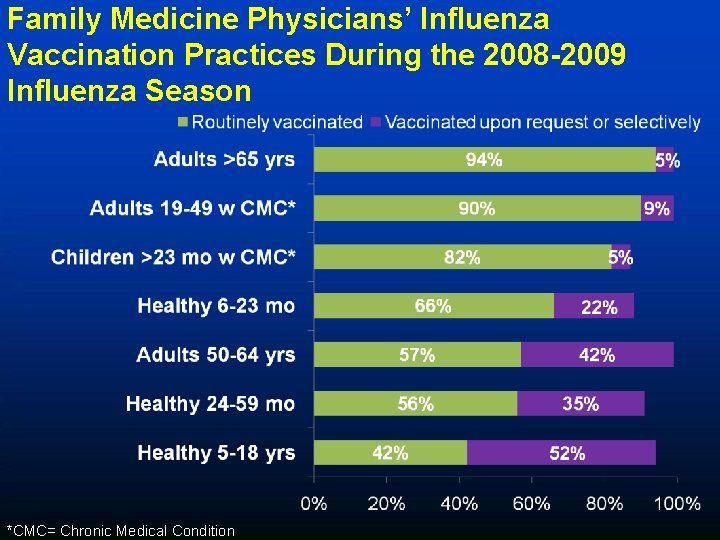
Family Medicine Physicians’ Influenza Vaccination Practices During the 2008 -2009 Influenza Season *CMC= Chronic Medical Condition

Barriers to Administering Both the New H 1 N 1 and the Seasonal Influenza Vaccines Peds FM GIM Significant % Moderate % If 3 or more visits are required to administer all doses of both vaccines 38 38 36 32 29 32 Parent unwillingness to allow their child to receive up to 4 different injections 34 43 30 42 -- -- Patient or parental concerns about the safety of the new H 1 N 1 vaccine 35 42 24 43 20 40 Lack of data about the efficacy of the new H 1 N 1 vaccine 25 44 19 32 22 37 Barrier

Barriers to Administering Both the New H 1 N 1 and the Seasonal Influenza Vaccines Peds FM GIM Significant % Moderate % Physician concern about the safety of the new H 1 N 1 vaccine 15 35 14 26 14 25 If the vaccine had to be given under an Emergency Use Agreement requiring physicians to give patients a fact sheet and report basic information to the FDA 14 23 8 27 10 28 Inadequate storage space for both vaccines 10 19 6 16 5 14 Physician perception that the new H 1 N 1 vaccine is a necessary vaccine 2 10 2 8 Unwillingness of adult patients to receive two different influenza vaccines -- -- 14 39 18 38 Barrier

Preference for Receiving Public Health. Related Information about Seasonal or H 1 N 1 Influenza Vaccines from a Reliable Source (CDC/ACIP) *Initiated by the CDC (Twitter, Facebook, blogs, RSS feeds, Widgets, e-health briefs, etc)