Childhood Immunization Update DPH 2019 Audience Presenters Name















































- Slides: 74

Childhood Immunization Update DPH 2019 Audience / Presenter’s Name / Date GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Disclosure Statements • Neither the planners of this session nor I have any financial relationship with pharmaceutical companies, biomedical device manufacturers, or corporations whose products and services are related to the vaccines we discuss • There is no commercial support being received for this event • The mention of specific brands of vaccines in this presentation is for the purpose of providing education and does not constitute endorsement • The GA Immunization Program utilizes ACIP recommendations as the basis for this presentation and for our guidelines, policies, and recommendations • For certain vaccines this may represent a slight departure from or off-label use of the vaccine package insert guidelines GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Disclosure Statements • To obtain nursing contact hours for this session, you must be present for the entire session and complete an evaluation. • Continuing Education credit will be provided through the Georgia Department of Public Health • Georgia Department of Public Health is an approved provider of continuing nursing education by the Alabama State Nurses Association, an accredited approver by the American Nurses Credentialing Center’s Commission of Accreditation GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Objectives At the end of this presentation, participants will be able to: Recall the role vaccines have played in preventing diseases Discuss the importance of vaccines for children Recall two recent immunization updates Discuss the role of a vaccine champion Discuss GA Immunization law and DPH rules and regulations for schools and child care attendance • List at least two reliable sources for immunization information • • • GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Topics for Discussion • 2019 Immunization Schedule Changes • ACIP Recommendations/Updates • New and future vaccines for potential use in practice GEORGIA DEPARTME NT OF PUBLIC HE ALTH

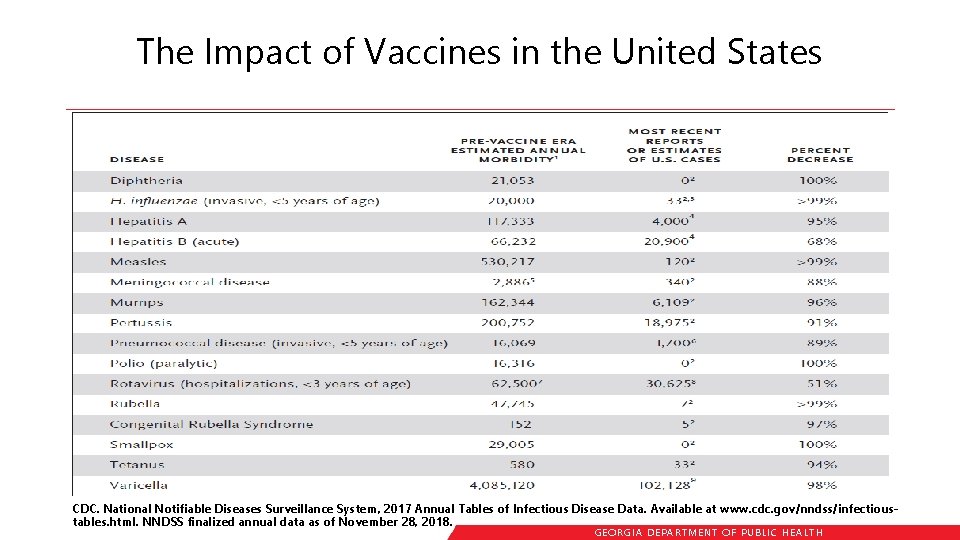
The Impact of Vaccines in the United States CDC. National Notifiable Diseases Surveillance System, 2017 Annual Tables of Infectious Disease Data. Available at www. cdc. gov/nndss/infectioustables. html. NNDSS finalized annual data as of November 28, 2018. GEORGIA DEPARTME NT OF PUBLIC HE ALTH

VPD Vaccination Rate Needed for Herd Immunity Measles 92 -94% Pertussis 92 -94% Diphtheria 83 -85% Rubella 83 -85% Mumps 75 -86% Influenza 30 -75% MMWR. 2017 Nov 3; 66(43): 1171– 1177 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

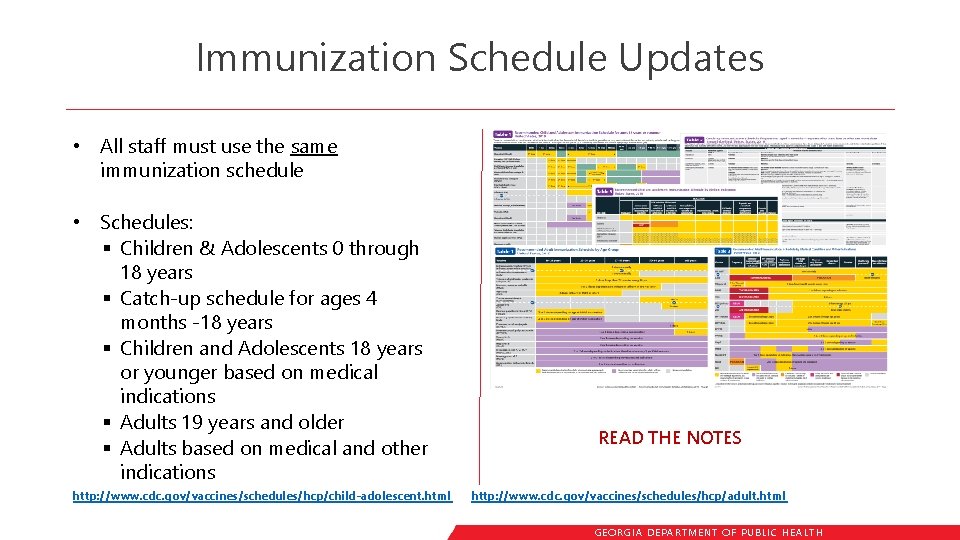
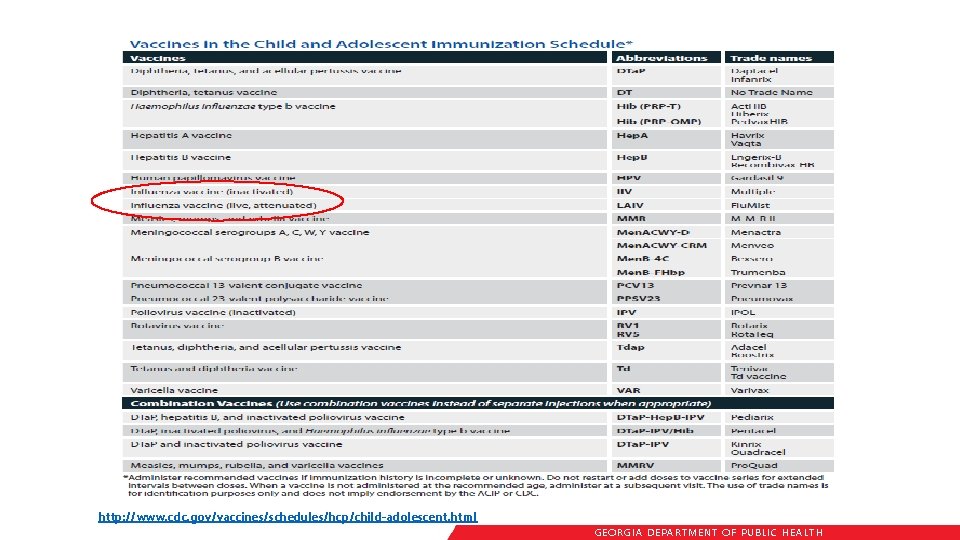
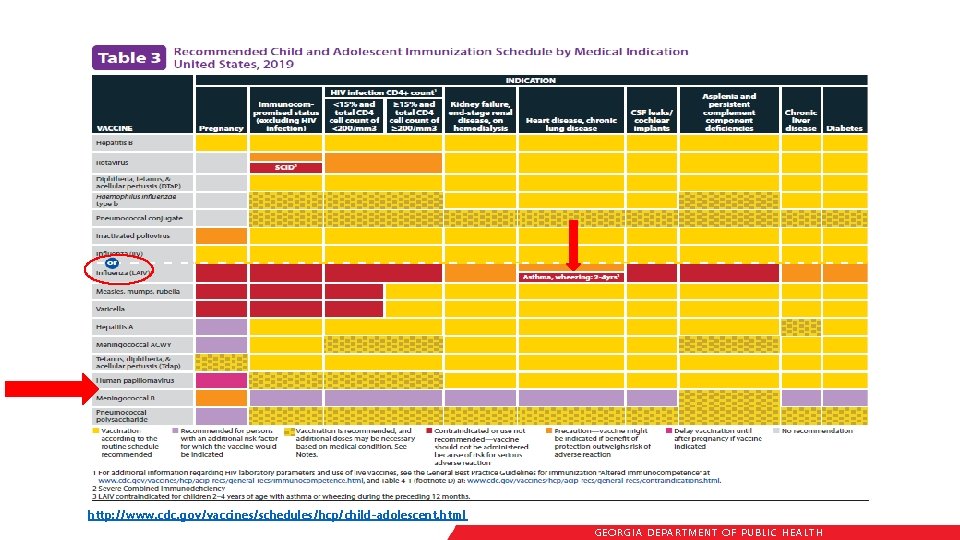
Immunization Schedule Updates • All staff must use the same immunization schedule • Schedules: § Children & Adolescents 0 through 18 years § Catch-up schedule for ages 4 months -18 years § Children and Adolescents 18 years or younger based on medical indications § Adults 19 years and older § Adults based on medical and other indications http: //www. cdc. gov/vaccines/schedules/hcp/child-adolescent. html READ THE NOTES http: //www. cdc. gov/vaccines/schedules/hcp/adult. html GEORGIA DEPARTME NT OF PUBLIC HE ALTH

http: //www. cdc. gov/vaccines/schedules/hcp/child-adolescent. html GEORGIA DEPARTME NT OF PUBLIC HE ALTH

http: //www. cdc. gov/vaccines/schedules/hcp/child-adolescent. html GEORGIA DEPARTME NT OF PUBLIC HE ALTH

http: //www. cdc. gov/vaccines/schedules/hcp/child-adolescent. html GEORGIA DEPARTME NT OF PUBLIC HE ALTH

http: //www. cdc. gov/vaccines/schedules/hcp/child-adolescent. html GEORGIA DEPARTME NT OF PUBLIC HE ALTH

What Does It All Mean? Indication -Information about the appropriate use of the vaccine Recommendation -ACIP statement that broadens and further delineates the Indication found in the package insert -Basis for standards for best practice Requirement -Mandate by a state that a particular vaccine must be administered and documented before entrance to child care and/or school GEORGIA DEPARTME NT OF PUBLIC HE ALTH

General Best Practice Guidelines • Timing and Spacing of Immunobiologics • Contraindications and Precautions • Preventing and Managing Adverse Reactions • Vaccine Administration • Storage and Handling of Immunobiologics • Altered Immunocompetence • Special Situations • Vaccination Records • Vaccination Programs • Vaccine Information Sources GEORGIA DEPARTME NT OF PUBLIC HE ALTH

General Best Practice Updates May 14, 2018 • Timing and Spacing, LAIV added, MPSV 23 removed July 18, 2018 • Hep A/ IG administration changed • Three precautions removed from DTa. P row • Varicella updated for the use of aspirin or aspirin-containing products • table 4 -2 under contraindications and precautions, header changed to conditions • “Multiple vaccinations”, Hep. A and IG- this couplet added to the pairs which should not be administered in the same limb • Vaccine Administration, RZV Row/Dose Column state that only 0. 5 cc should be withdrawn even if more vaccine remains in the vial https: //www. cdc. gov/vaccines/hcp/acip-recs/general-recs-errata. html GEORGIA DEPARTME NT OF PUBLIC HE ALTH

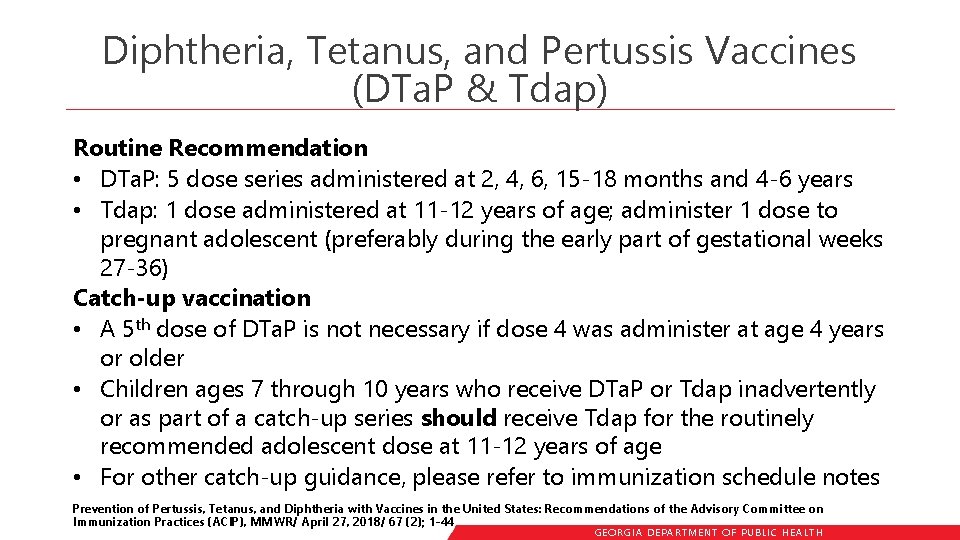
Diphtheria, Tetanus, and Pertussis Vaccines (DTa. P & Tdap) Routine Recommendation • DTa. P: 5 dose series administered at 2, 4, 6, 15 -18 months and 4 -6 years • Tdap: 1 dose administered at 11 -12 years of age; administer 1 dose to pregnant adolescent (preferably during the early part of gestational weeks 27 -36) Catch-up vaccination • A 5 th dose of DTa. P is not necessary if dose 4 was administer at age 4 years or older • Children ages 7 through 10 years who receive DTa. P or Tdap inadvertently or as part of a catch-up series should receive Tdap for the routinely recommended adolescent dose at 11 -12 years of age • For other catch-up guidance, please refer to immunization schedule notes Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP), MMWR/ April 27, 2018/ 67 (2); 1 -44 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

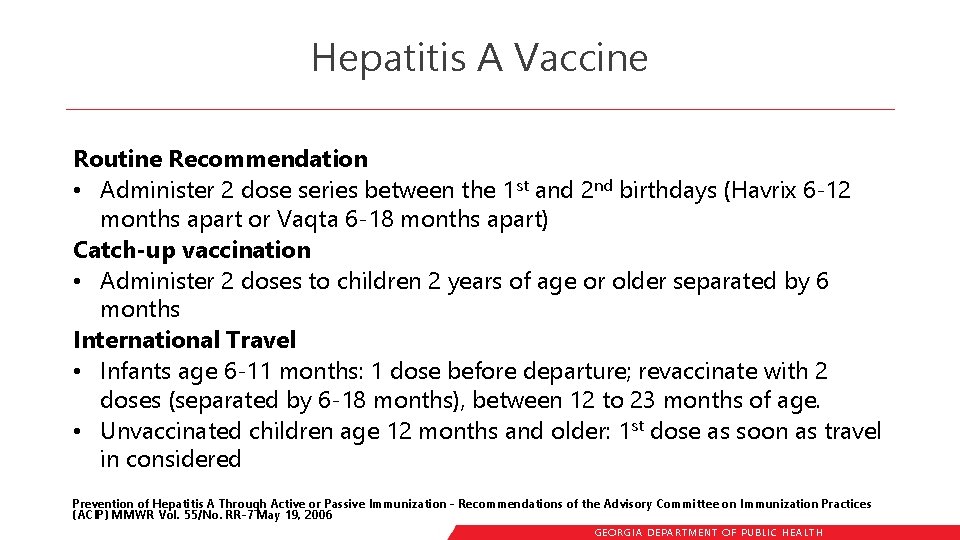
Hepatitis A Vaccine Routine Recommendation • Administer 2 dose series between the 1 st and 2 nd birthdays (Havrix 6 -12 months apart or Vaqta 6 -18 months apart) Catch-up vaccination • Administer 2 doses to children 2 years of age or older separated by 6 months International Travel • Infants age 6 -11 months: 1 dose before departure; revaccinate with 2 doses (separated by 6 -18 months), between 12 to 23 months of age. • Unvaccinated children age 12 months and older: 1 st dose as soon as travel in considered Prevention of Hepatitis A Through Active or Passive Immunization - Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Vol. 55/No. RR-7 May 19, 2006 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Hepatitis A Vaccine Special Situations • Administer a 2 dose series to at risk population § Chronic liver disease, clotting factor disorders, MSM, injection and non injection drug users, homelessness, work with Hep. A virus (i. e. in research labs or nonhuman primates with Hep. A infection) § Persons who anticipate close, personal contact with an international adoptee during the first 60 days after arrival in the U. S. from a country with high or intermediate endemicity (administer the 1 st dose as soon as the adoption is planned, at least 2 weeks before the adoptee’s arrival) • Administer for post-exposure for all persons age 12 months or older § Hep A vaccine or IG may be administered to persons age 40 years or older, depending on the provider’s risk assessment MMWR/ February 15, 2019 / 68(6); 153– 156 MMWR November 2, 2018/ 67(43); 1208– 1210 MMWR 2018/ 67(43); 1216 -1220 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Hepatitis B Vaccine Routine Recommendation • Dose 1 @ birth • Dose 2 @ 1 -2 months of age § at least 1 month after first dose • Dose 3 @ 6 -18 months of age § minimum of 4 months after the first dose § minimum of 2 months after the second dose but not before an infant is 24 weeks of age • Administration of 4 doses is permitted when a combination vaccine containing Hep. B is used after the birth dose GEORGIA DEPARTME NT OF PUBLIC HE ALTH

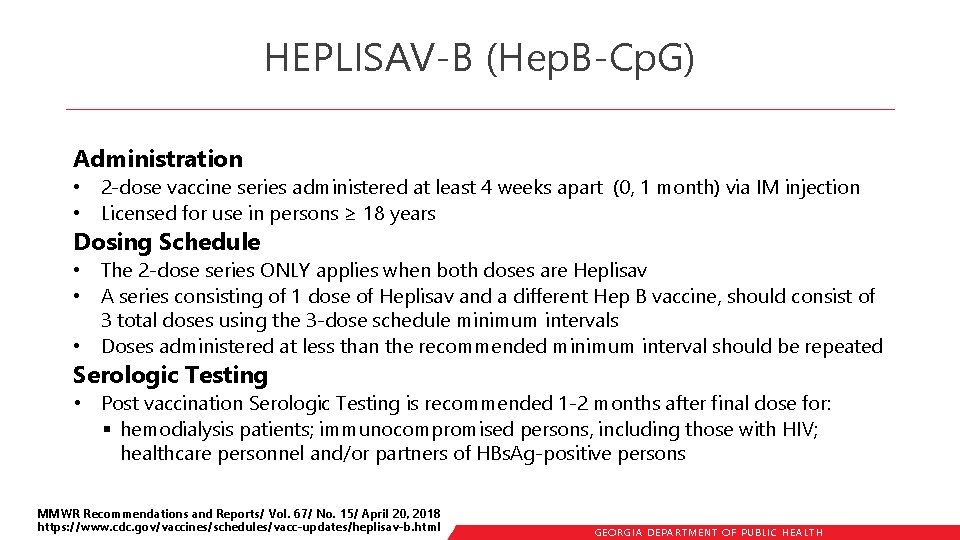
HEPLISAV-B (Hep. B-Cp. G) Administration • 2 -dose vaccine series administered at least 4 weeks apart (0, 1 month) via IM injection • Licensed for use in persons ≥ 18 years Dosing Schedule • The 2 -dose series ONLY applies when both doses are Heplisav • A series consisting of 1 dose of Heplisav and a different Hep B vaccine, should consist of 3 total doses using the 3 -dose schedule minimum intervals • Doses administered at less than the recommended minimum interval should be repeated Serologic Testing • Post vaccination Serologic Testing is recommended 1 -2 months after final dose for: § hemodialysis patients; immunocompromised persons, including those with HIV; healthcare personnel and/or partners of HBs. Ag-positive persons MMWR Recommendations and Reports/ Vol. 67/ No. 15/ April 20, 2018 https: //www. cdc. gov/vaccines/schedules/vacc-updates/heplisav-b. html GEORGIA DEPARTME NT OF PUBLIC HE ALTH

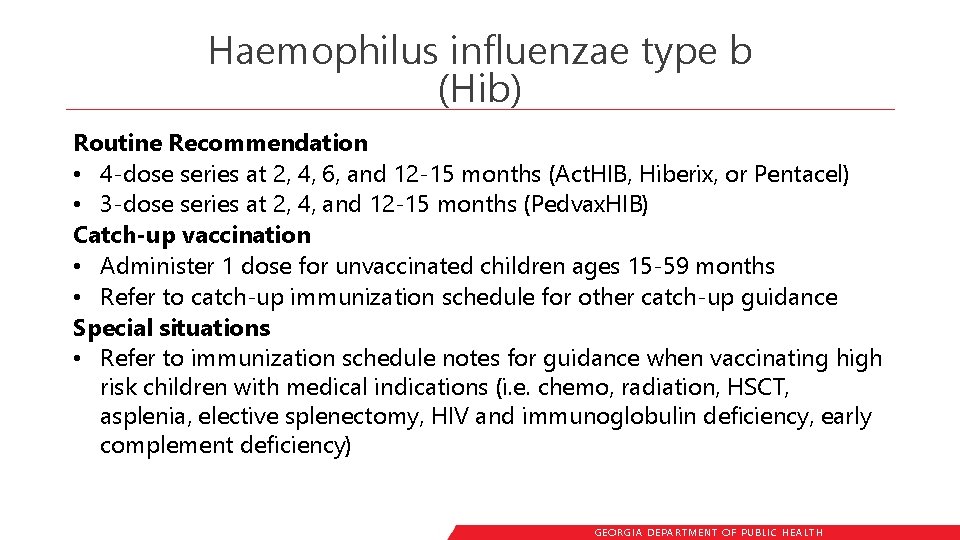
Haemophilus influenzae type b (Hib) Routine Recommendation • 4 -dose series at 2, 4, 6, and 12 -15 months (Act. HIB, Hiberix, or Pentacel) • 3 -dose series at 2, 4, and 12 -15 months (Pedvax. HIB) Catch-up vaccination • Administer 1 dose for unvaccinated children ages 15 -59 months • Refer to catch-up immunization schedule for other catch-up guidance Special situations • Refer to immunization schedule notes for guidance when vaccinating high risk children with medical indications (i. e. chemo, radiation, HSCT, asplenia, elective splenectomy, HIV and immunoglobulin deficiency, early complement deficiency) GEORGIA DEPARTME NT OF PUBLIC HE ALTH

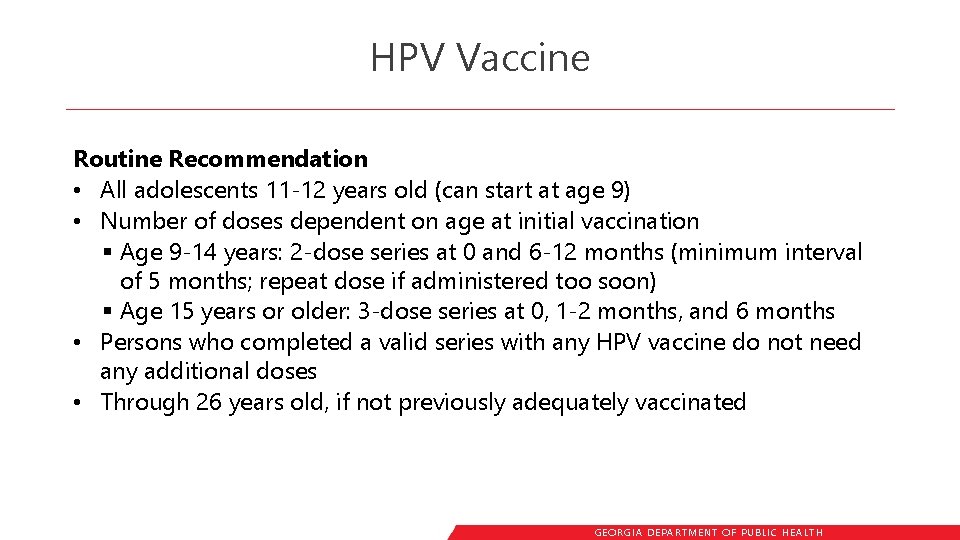
HPV Vaccine Routine Recommendation • All adolescents 11 -12 years old (can start at age 9) • Number of doses dependent on age at initial vaccination § Age 9 -14 years: 2 -dose series at 0 and 6 -12 months (minimum interval of 5 months; repeat dose if administered too soon) § Age 15 years or older: 3 -dose series at 0, 1 -2 months, and 6 months • Persons who completed a valid series with any HPV vaccine do not need any additional doses • Through 26 years old, if not previously adequately vaccinated GEORGIA DEPARTME NT OF PUBLIC HE ALTH

HPV Vaccine for Special Population and Situations Special situations • Immunocompromised: aged 9 -26 years, administer 3 -dose series • History of sexual abuse or assault: begin series at age 9 • Pregnancy: vaccine is not recommended during pregnancy § If administered inadvertently while pregnant, no intervention needed-delay further doses until after pregnancy § Pregnancy testing not needed before vaccination GEORGIA DEPARTME NT OF PUBLIC HE ALTH

GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Influenza Vaccines for 2018 -2019 Season in the U. S. • Trivalent Vaccines (IIV 3): § A/Michigan/45/2015 (H 1 N 1) § A/Singapore/INFIMH-16 -0019/2016 (H 3 N 2)-like virus (NEW) § B/Colorado/06/2017 -like virus (Victoria lineage)- like virus (NEW) • Quadrivalent Vaccines (IIIV 4) will also include: § B/Phuket/3073/2013 -like virus (Yamagata lineage)- like virus • ACIP recommends annual influenza vaccine for all persons 6 months of age and older who do not have contraindications Recommendations and Reports Vol. 67 / No. 3 MMWR / August 24, 2018 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

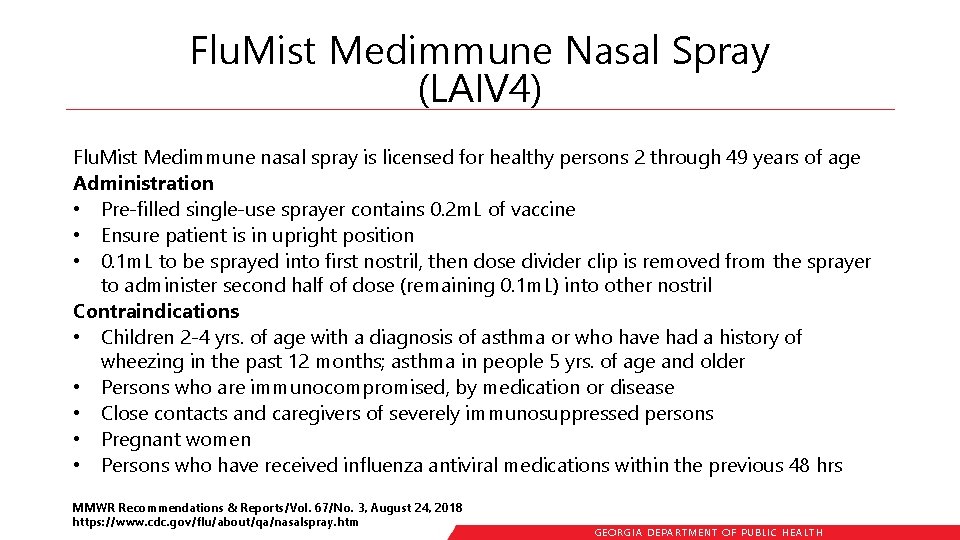
Flu. Mist Medimmune Nasal Spray (LAIV 4) Flu. Mist Medimmune nasal spray is licensed for healthy persons 2 through 49 years of age Administration • Pre-filled single-use sprayer contains 0. 2 m. L of vaccine • Ensure patient is in upright position • 0. 1 m. L to be sprayed into first nostril, then dose divider clip is removed from the sprayer to administer second half of dose (remaining 0. 1 m. L) into other nostril Contraindications • Children 2 -4 yrs. of age with a diagnosis of asthma or who have had a history of wheezing in the past 12 months; asthma in people 5 yrs. of age and older • Persons who are immunocompromised, by medication or disease • Close contacts and caregivers of severely immunosuppressed persons • Pregnant women • Persons who have received influenza antiviral medications within the previous 48 hrs MMWR Recommendations & Reports/Vol. 67/No. 3, August 24, 2018 https: //www. cdc. gov/flu/about/qa/nasalspray. htm GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Product Updates FDA licensure and labeling changes: • January 23, 2019 FDA approved use of the 0. 5 m. L dose of Sanofi’s Fluzone Quadrivalent influenza vaccine to include children age 6 through 35 months • Approval of Afluria Quadrivalent (Seqirus) and Flublok Quadrivalent (Protein Sciences) • Expansion of the age indication for Flu. Laval Quadrivalent (GSK) and Fluarix Quadrivalent (GSK) to age 6 months and older (previously licensed for persons 3 years and older) • Expansion of the age indication for Afluria (Seqirus) to include persons 5 years and older (previously recommended for persons 18 years and older) • CDC published ACIP’s recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV 4) in the 2018 -19 influenza season GEORGIA DEPARTME NT OF PUBLIC HE ALTH

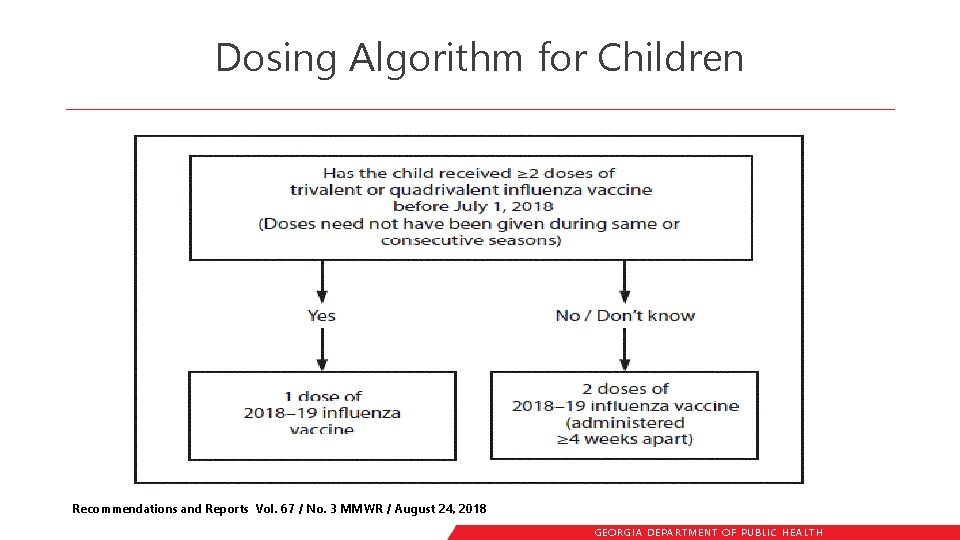
Dosing Algorithm for Children Recommendations and Reports Vol. 67 / No. 3 MMWR / August 24, 2018 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Measles, Mumps, Rubella Routine Recommendation • 2 - dose series at ages 12 through 15 months and 4 through 6 years (dose 2 may be given as early as 4 weeks after the 1 st dose) Catch-up vaccination • Unvaccinated children and adolescents: 2 doses at least 4 weeks apart Special situations • International travel: 1 dose prior to departure for infants ages 6 -11 months followed by routine 2 dose series at 12 -15 months (12 months for children in high-risk areas) and dose 2 as early as 4 weeks • Administer a 2 dose series at least 4 weeks apart for unvaccinated children 12 months and older, prior to departure GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Varicella Routine Recommendation • 2 -dose series at 12 through 15 months and 4 through 6 years • The 2 nd dose may be given as early as 3 months after the 1 st dose (a dose given after a 4 -week interval may be counted) Catch-up vaccination • Administer 2 doses to persons 7 -18 years without evidence of immunity • Ages 7 -12 years routine interval between doses 3 months (minimum interval: 4 weeks) • Ages 13 years and older minimum interval 4 weeks between doses • The maximum age for use of MMRV is 12 years old GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Varicella Immunity ACIP considers evidence of immunity to varicella to be: • Documentation of 2 doses of vaccine given no earlier than age 12 months, with at least 3 months between doses for children younger than age 13 years, or at least 4 weeks between doses for people age 13 years and older • U. S. -born before 1980* (year of birth is not considered as evidence of immunity for healthcare personnel, and pregnant women) • A healthcare provider's diagnosis of herpes zoster, varicella or history of varicella disease • Laboratory evidence of immunity to or confirmation of disease MMWR 2007; 56(RR-4); 16 -17 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

MMRV (Pro. Quad®) Routine Recommendation • May be administered to children 12 months through 12 years of age • Maximum age for MMRV use is 12 years old GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Spacing of Live Virus Vaccines and Other Products • PPD and live virus vaccine § Apply PPD at same visit as MMR § If MMR given first, delay PPD 4 weeks or longer if not given during the same visit § If PPD given first, administer MMR when client returns for skin test reading • Spacing with antibody-containing products such as immune globulin (IG) with live vaccines MMWR 2007; 56(RR-4); 16 -17 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Polio Routine Recommendation • 4 - dose series at 2, 4, 6 through 18 months, and 4 through 6 years • Final dose after the fourth birthday and at least 6 months after the previous dose • 4 or more doses of IPV can be administered before the 4 th birthday when a combination vaccine containing IPV is used. However, a final dose after the 4 th birthday is still recommended Catch-up vaccination • Final dose to be given on or after the 4 th birthday and at least 6 months after the previous dose, regardless of the number of previous doses • IPV is not routinely recommended for U. S. residents 18 years and older In the first six months of life, use minimum ages and intervals only for travel to a polio-endemic region or during an outbreak GEORGIA DEPARTME NT OF PUBLIC HE ALTH

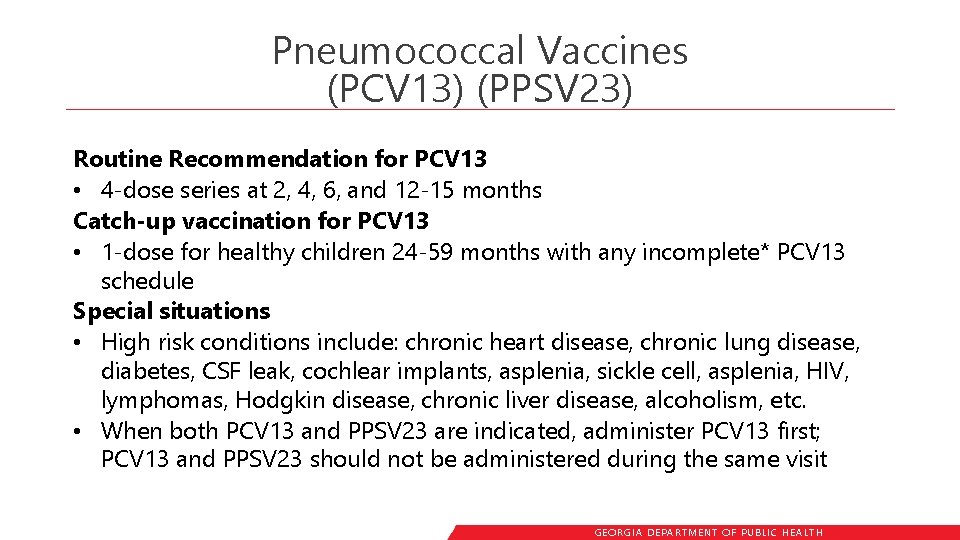
Pneumococcal Vaccines (PCV 13) (PPSV 23) Routine Recommendation for PCV 13 • 4 -dose series at 2, 4, 6, and 12 -15 months Catch-up vaccination for PCV 13 • 1 -dose for healthy children 24 -59 months with any incomplete* PCV 13 schedule Special situations • High risk conditions include: chronic heart disease, chronic lung disease, diabetes, CSF leak, cochlear implants, asplenia, sickle cell, asplenia, HIV, lymphomas, Hodgkin disease, chronic liver disease, alcoholism, etc. • When both PCV 13 and PPSV 23 are indicated, administer PCV 13 first; PCV 13 and PPSV 23 should not be administered during the same visit GEORGIA DEPARTME NT OF PUBLIC HE ALTH

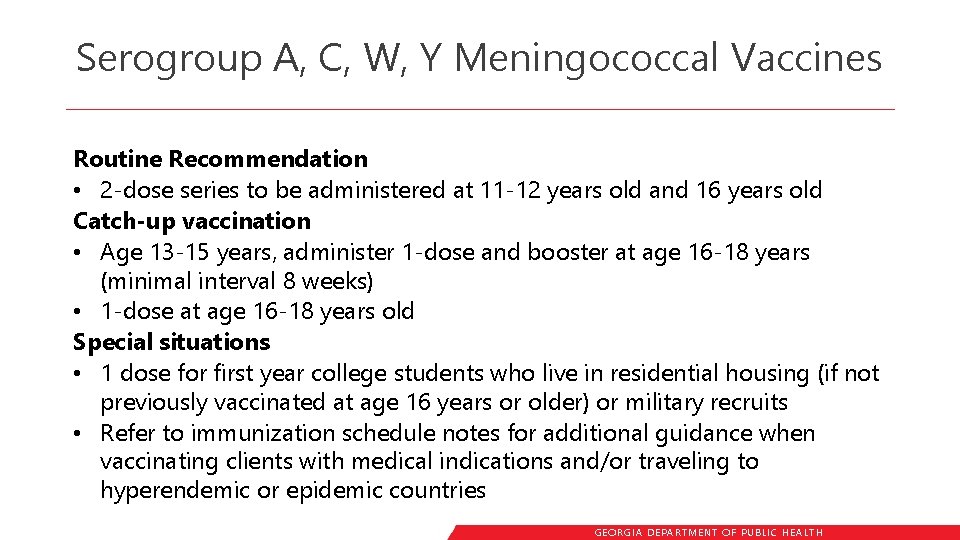
Serogroup A, C, W, Y Meningococcal Vaccines Routine Recommendation • 2 -dose series to be administered at 11 -12 years old and 16 years old Catch-up vaccination • Age 13 -15 years, administer 1 -dose and booster at age 16 -18 years (minimal interval 8 weeks) • 1 -dose at age 16 -18 years old Special situations • 1 dose for first year college students who live in residential housing (if not previously vaccinated at age 16 years or older) or military recruits • Refer to immunization schedule notes for additional guidance when vaccinating clients with medical indications and/or traveling to hyperendemic or epidemic countries GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Serogroup B Meningococcal Vaccines Clinical discretion • May be given at clinical discretion to adolescents 16 -23 years who are not at increased risk (preferred age 16 -18 years) § Bexsero: 2 doses at least 1 month apart § Trumenba: 2 doses at least 6 months apart. If 2 nd dose given earlier than 6 months, give 3 rd dose at least 4 months after the 2 nd dose Special situations • Anatomic or functional asplenia, sickle cell disease, persistent complement component deficiency (including eculizumab use) § Bexsero: 2 -doses at least 1 month apart § Trumenba: 3 -dose series at 0, 1 -2, and 6 months • Bexsero and Trumenba are not interchangeable GEORGIA DEPARTME NT OF PUBLIC HE ALTH

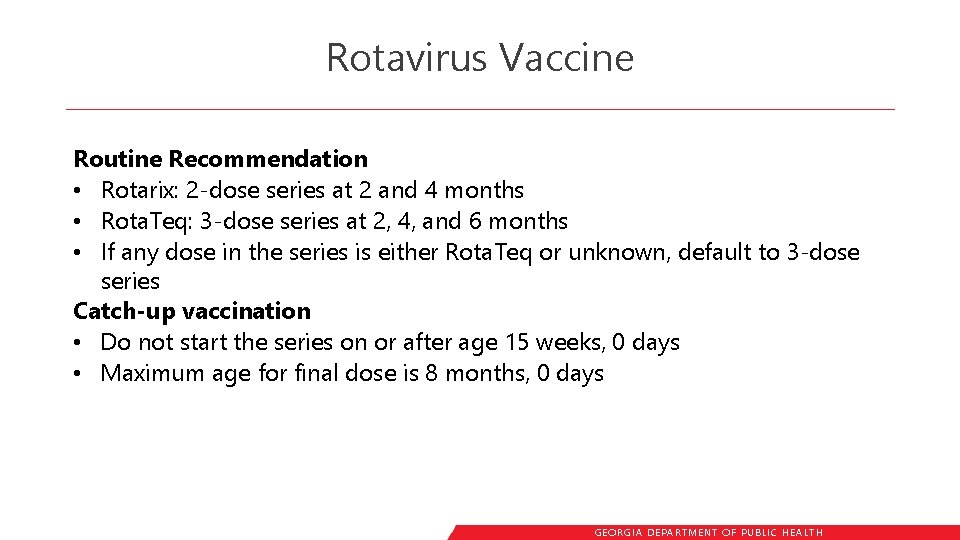
Rotavirus Vaccine Routine Recommendation • Rotarix: 2 -dose series at 2 and 4 months • Rota. Teq: 3 -dose series at 2, 4, and 6 months • If any dose in the series is either Rota. Teq or unknown, default to 3 -dose series Catch-up vaccination • Do not start the series on or after age 15 weeks, 0 days • Maximum age for final dose is 8 months, 0 days GEORGIA DEPARTME NT OF PUBLIC HE ALTH

GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Just as a reminder…… Regardless of: • the availability of vaccine • the funding of the vaccine (VFC, state-supplied, or private stock) • whether the vaccine is required for school or child care or not………. FOLLOW ACIP Recommendations!!! GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Test Your Knowledge! Q: We have adolescents in our practice who have received the first 2 doses of the HPV series 1 or 2 months apart according to the 3 -dose schedule. Can we consider their HPV vaccine series to be complete or do we need to give these patients a third dose? A: People who have received 2 doses of HPV vaccine separated by less than 5 months should receive a third dose 6– 12 months after dose #1 and at least 12 weeks after dose #2. GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Test Your Knowledge! Q: Which patients should receive a 2 -dose schedule of Trumenba (Men. B, Pfizer)? A: Healthy adolescents who are not at increased risk for meningococcal disease should receive 2 doses of Trumenba administered at 0 and 6 months. If the second dose is given at an interval of less than 6 months, a third dose should be given at least 4 months after the 2 nd dose. Recommended Immunization Schedule for Children and Adolescents Aged 18 years or younger, United States, 2017 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Test Your Knowledge! Q: If someone received MPSV 4 or Men. ACWY at age 9 years, will two additional doses of Men. ACWY be needed? A: Yes. Doses of quadrivalent meningococcal vaccine (either MPSV 4 or Men. ACWY) given before 10 years of age should not be counted as part of the routine 2 -dose series. If a child received a dose of either MPSV 4 or Men. ACWY before age 10 years, they should receive a dose of Men. ACWY at 11 or 12 years and a booster dose at age 16 years. Recommended Immunization Schedule for Children and Adolescents Aged 18 years or younger, United States, 2017 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Requirements for School and Childcare Attendance GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Goal • Vaccines work Goal 100 % compliance rate • Immunization Laws work • Partnerships work GEORGIA DEPARTME NT OF PUBLIC HE ALTH

JOB AIDS GEORGIA DEPARTME NT OF PUBLIC HE ALTH

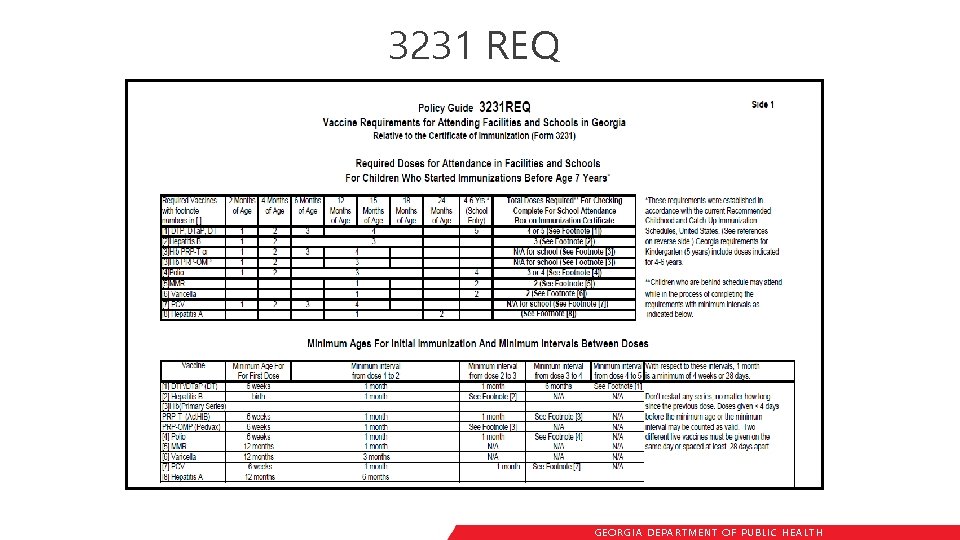
3231 REQ GEORGIA DEPARTME NT OF PUBLIC HE ALTH

3231 INS GEORGIA DEPARTME NT OF PUBLIC HE ALTH

School Requirement Updates • 3231 INS updated December 2017 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

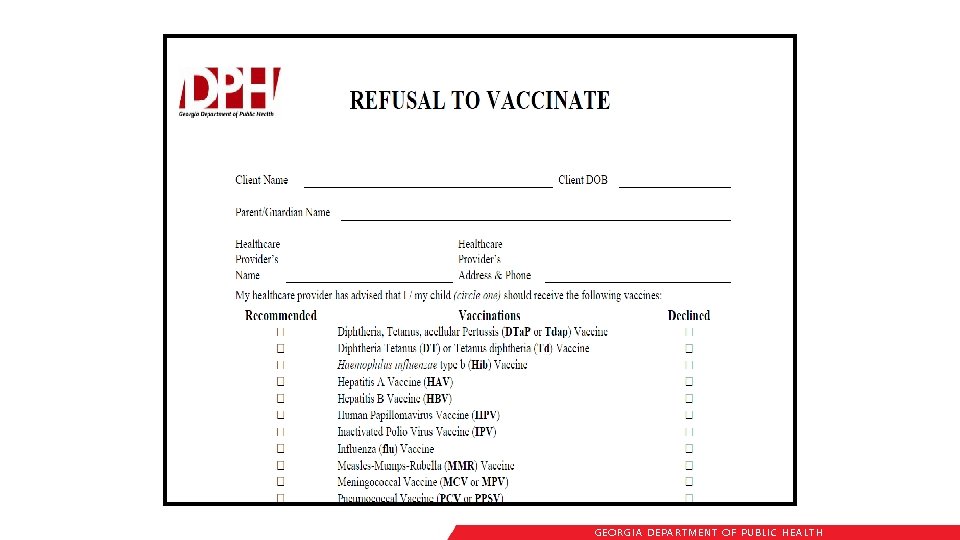
Certificate of Immunization (Form 3231) • Certificate on file at each facility or school • Photocopies acceptable • A licensed Georgia physician, APRN, PA or public health official is responsible for completing the certificate • Only physician offices and health clinics can obtain blank certificates GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Valid Certificates All certificates must be marked with: • Child’s name • Birth date • Name and Address of Physician, APRN, PA, Qualified Board of Health official or State Immunization Office Official • Certified Signature • Date of Issue GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Expiration Date Expires on the date entered as “Expiration Date” Must be replaced with a current certificate within 30 days Required for all children less than age four years Required for all children ages four through ten years who have not completed K through 6 th grade requirements or children 10 years and older who have not completed 7 th grade or higher requirements • Required if a medical exemption for a vaccine(s) is marked • • GEORGIA DEPARTME NT OF PUBLIC HE ALTH

“Complete for School Attendance” • Issued only to children who: § Are four years of age or older; and § Have met all the requirements for school attendance as outlined in the Policy Guide 3231 REQ; and § Have all the required vaccine administration dates or natural immunity dates filled in; and § Do not have a “Date of Expiration” GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Exemptions Medical • Physical disability or condition • Documented in the medical exemption box indicated for each vaccine • Reviewed annually GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Exemptions Religious • Documented on form 2208 • Form kept on file by the school or facility in lieu of a Certificate of Immunization (form 3231) • Do not expire GEORGIA DEPARTME NT OF PUBLIC HE ALTH

School Requirement Updates • DPH Rules and Regulations 511 -2 -2 -. 07 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Child Care Requirements • Number of vaccine doses • Always need more doses • Must have a current “expiration date” GEORGIA DEPARTME NT OF PUBLIC HE ALTH

School Requirements • Any “new entrant” enrolling in a Georgia school at any grade or level, must be age appropriately immunized with required vaccines • Number of doses depends on the child’s age • “Complete for 7 th Grade or higher ” is marked; certificate is complete GEORGIA DEPARTME NT OF PUBLIC HE ALTH

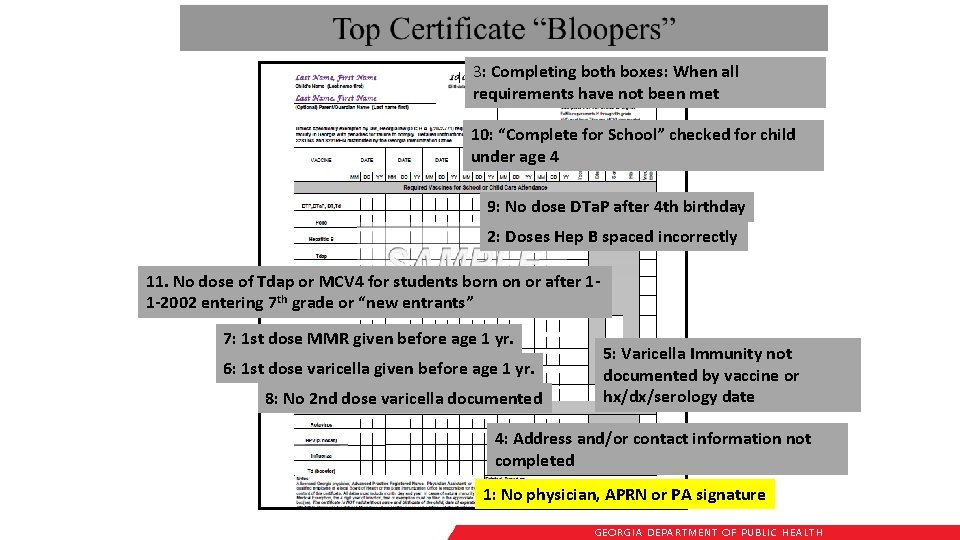
3: Completing both boxes: When all requirements have not been met 10: “Complete for School” checked for child under age 4 9: No dose DTa. P after 4 th birthday 2: Doses Hep B spaced incorrectly 11. No dose of Tdap or MCV 4 for students born on or after 11 -2002 entering 7 th grade or “new entrants” 7: 1 st dose MMR given before age 1 yr. 6: 1 st dose varicella given before age 1 yr. 8: No 2 nd dose varicella documented 5: Varicella Immunity not documented by vaccine or hx/dx/serology date 4: Address and/or contact information not completed 1: No physician, APRN or PA signature GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Filing of Certificates • Available for inspection by health officials • Photocopy acceptable • Sent copy to the new school/facility • In the case of religious exemption, form 2208 must be on file in lieu of form 3231 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Tickler Filing System Instructions located in the Immunization Guidelines for Child Care Facility Operators & School Personnel (Form 3258) • Set up by month and year • Parent reminders • Summary of GA Immunization requirements • Document follow-up • Enforce requirements GEORGIA DEPARTME NT OF PUBLIC HE ALTH

GRITS GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Responsibilities • Physicians and Public Health Clinics • Child Care and School • Parent/Caregiver GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Become a Vaccine Champion!! Critical Elements • Appropriate storage and handling of all vaccines • Correct administration of vaccines • Education of patients and parents about vaccines • Every office and clinic needs a vaccine champion GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Vaccine Champion Key Characteristics • Lead your immunization team • Educate all staff about new vaccines and recommendations • Educate new staff about vaccine storage, handling, & administration • Initiate processes to improve immunization rates in your practice/facility • Assure immunizations of all staff are up-to-date GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Improve Access To Immunizations • Immunization only visits • Walk-ins for immunizations • Implement standing orders • Early, extended, or weekend hours • Mass vaccination clinics GEORGIA DEPARTME NT OF PUBLIC HE ALTH

VAERS Public Health Reports should be faxed or mailed to the State Immunization Program. Fax number (404)657 -1463 GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Vaccine Injury Compensation Program (VICP) The National Vaccine Injury Compensation Program provides compensation to individuals found to be injured by or have died from certain childhood vaccines • • Established in 1988 by NCVIA Federal “no fault” system to compensate those injured Claim must be filed by individual, parent or guardian Must show that injury is on “Vaccine Injury Table” GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Should We Report Vaccine Errors to VAERS? • The Vaccine Adverse Event Reporting System (VAERS) accepts all reports, including reports of vaccination errors • VAERS is primarily for monitoring adverse health events, and we encourage reporting of clinically significant adverse health events following vaccination • Using clinical judgment, healthcare professionals can decide whether or not to report a medical error. For example, a healthcare professional might choose to report a vaccination error if the error might pose a safety risk (e. g. , administering a live vaccine to an immunocompromised patient) or the error would be preventable with public health action or education GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Resources for Factual & Responsible Vaccine Information www. immunize. org GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Stay Current! Sign up for listserv sites which provide timely information pertinent to your practice • www. immunize. org/resources/emailnews. asp • • • AAP Newsletter CDC immunization websites (32 in all) CHOP Parents Pack Newsletter IAC Express Websites specific to particular vaccines GEORGIA DEPARTME NT OF PUBLIC HE ALTH

Internet Resources Georgia Department of Public Health l l http: //dph. georgia. gov/immunization-section https: //dph. georgia. gov/train-trainer CDC Immunization information l l https: //www. cdc. gov/vaccines/index. html Send your clinical vaccine questions to NIPINFO@cdc. gov CDC Flu information l https: //www. cdc. gov/flu/ Immunization Action Coalition l www. immunize. org GEORGIA DEPARTME NT OF PUBLIC HE ALTH

State Resources • GA Immunization Program Office § On call Help line: 404 -657 -3158 § GRITS Help Line: 1 -866 -483 -2958 § VFC Help Line: 1 -800 -848 -3868 § Website http: //dph. georgia. gov/immunization-section § Your local Immunization Regional Program Consultant (IRC) § Epidemiology: 1 -866 -782 -4584 • GA Chapter of the AAP • GA Academy of Family Physicians GEORGIA DEPARTME NT OF PUBLIC HE ALTH

It’s a Team Effort! High Immunization rates begin with a team designed plan! What can your team do to improve rates? GEORGIA DEPARTME NT OF PUBLIC HE ALTH