Childhood Immunization How Far Weve Come And How



































- Slides: 60

Childhood Immunization How Far We’ve Come And How Far We Have to Go Alan R. Hinman, MD, MPH Indiana Immunization Conference October 16 -17, 2007

Outline of presentation • Current status of childhood immunization in the US • How immunizations are financed in the US • Inequities in financing – Causes – Possible solutions • Further issues with adult immunization

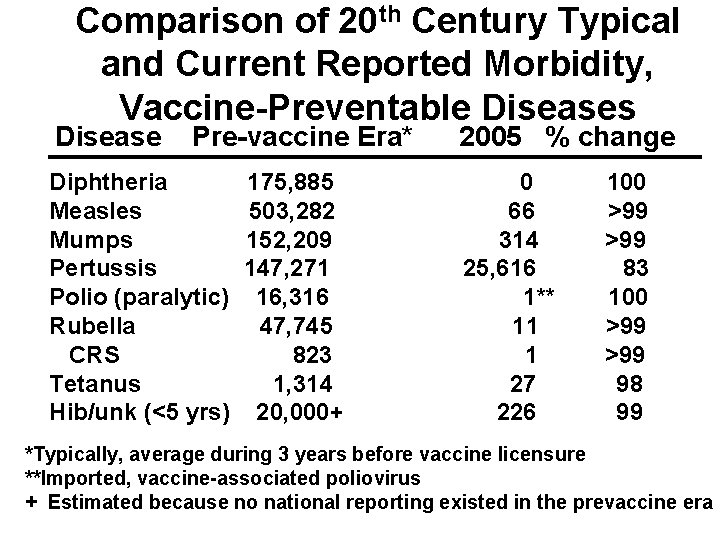
Comparison of 20 th Century Typical and Current Reported Morbidity, Vaccine-Preventable Diseases Disease Pre-vaccine Era* Diphtheria Measles Mumps Pertussis Polio (paralytic) Rubella CRS Tetanus Hib/unk (<5 yrs) 175, 885 503, 282 152, 209 147, 271 16, 316 47, 745 823 1, 314 20, 000+ 2005 % change 0 66 314 25, 616 1** 11 1 27 226 100 >99 83 100 >99 98 99 *Typically, average during 3 years before vaccine licensure **Imported, vaccine-associated poliovirus + Estimated because no national reporting existed in the prevaccine era

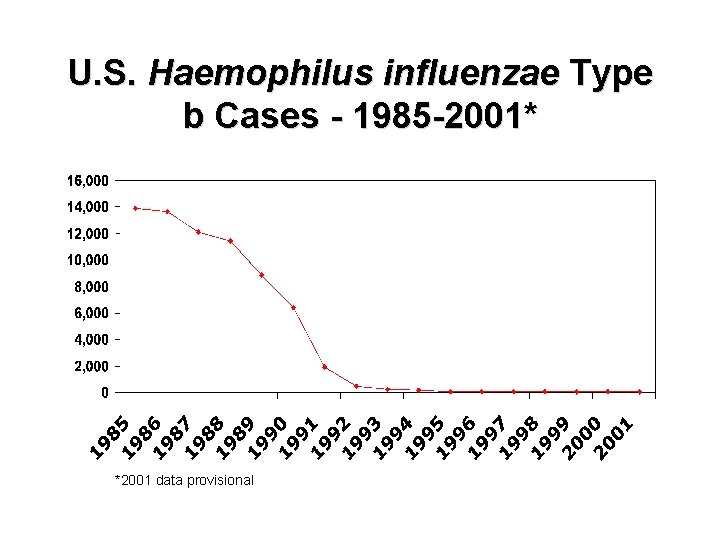
U. S. Haemophilus influenzae Type b Cases - 1985 -2001* *2001 data provisional

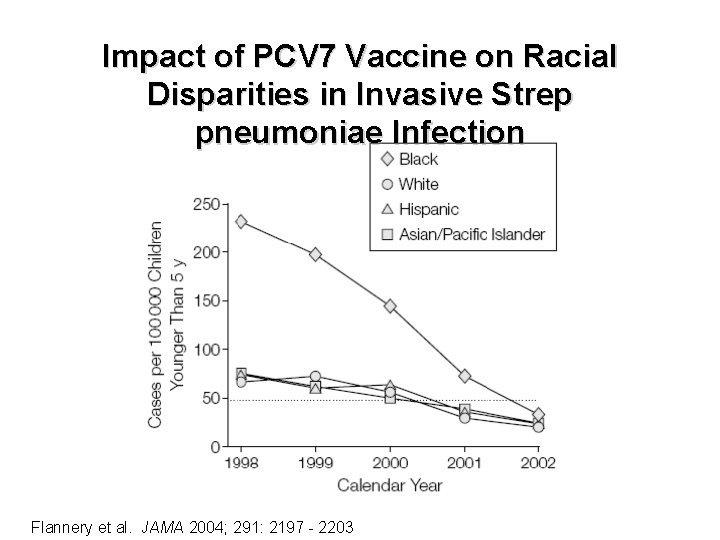
Impact of PCV 7 Vaccine on Racial Disparities in Invasive Strep pneumoniae Infection Flannery et al. JAMA 2004; 291: 2197 - 2203

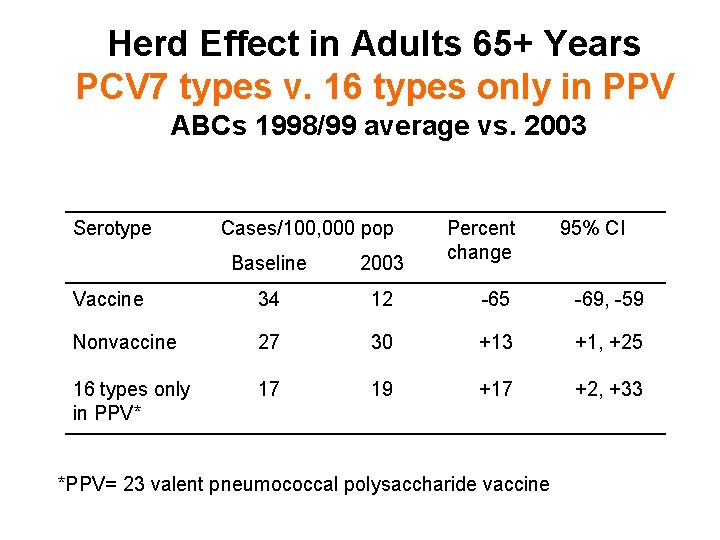
Herd Effect in Adults 65+ Years PCV 7 types v. 16 types only in PPV ABCs 1998/99 average vs. 2003 Serotype Cases/100, 000 pop Percent change 95% CI Baseline 2003 Vaccine 34 12 -65 -69, -59 Nonvaccine 27 30 +13 +1, +25 16 types only in PPV* 17 19 +17 +2, +33 *PPV= 23 valent pneumococcal polysaccharide vaccine

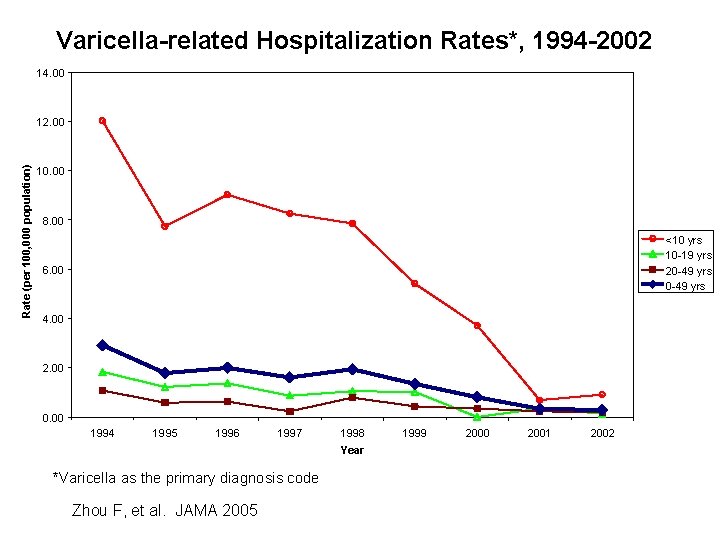
Varicella-related Hospitalization Rates*, 1994 -2002 14. 00 Rate (per 100, 000 population) 12. 00 10. 00 8. 00 <10 yrs 10 -19 yrs 20 -49 yrs 6. 00 4. 00 2. 00 0. 00 1994 1995 1996 1997 1998 Year *Varicella as the primary diagnosis code Zhou F, et al. JAMA 2005 1999 2000 2001 2002

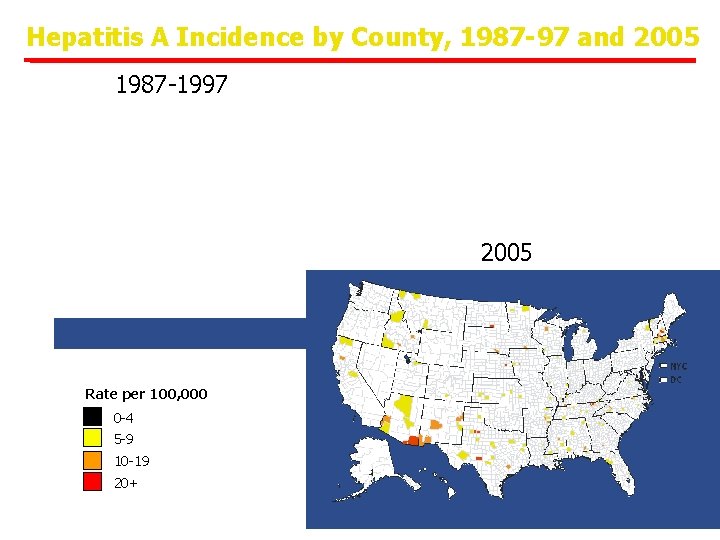
Hepatitis A Incidence by County, 1987 -97 and 2005 1987 -1997 2005 Rate per 100, 000 0 -4 5 -9 10 -19 20+

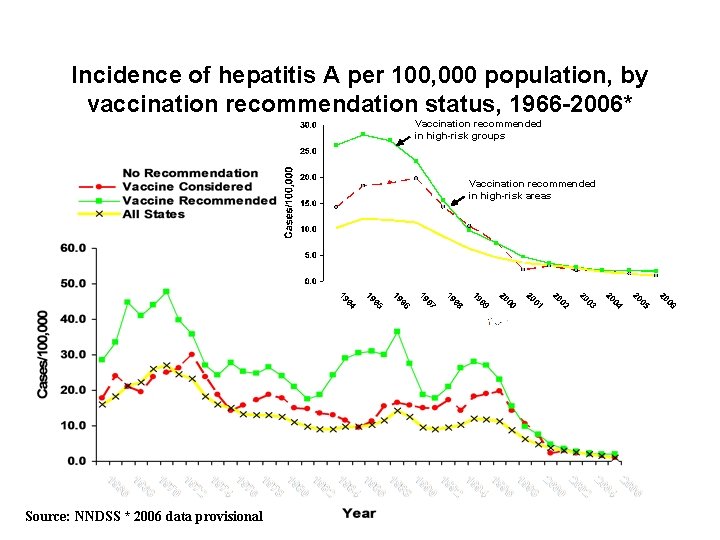
Incidence of hepatitis A per 100, 000 population, by vaccination recommendation status, 1966 -2006* Vaccination recommended in high-risk groups Vaccination recommended in high-risk areas Source: NNDSS * 2006 data provisional

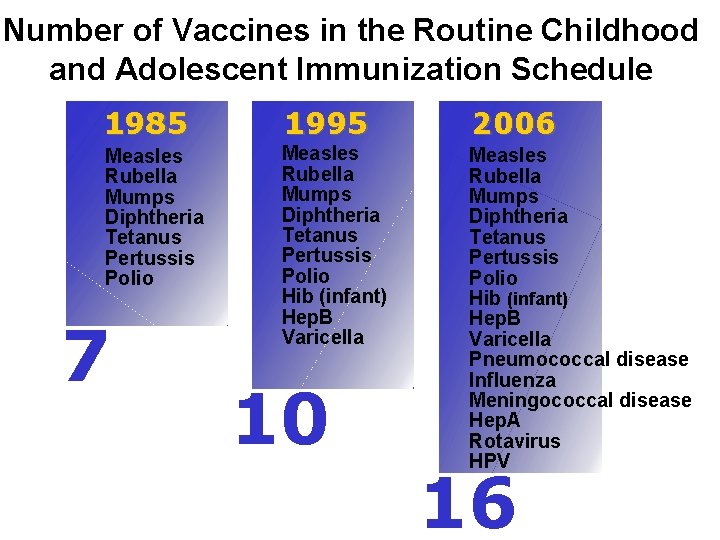
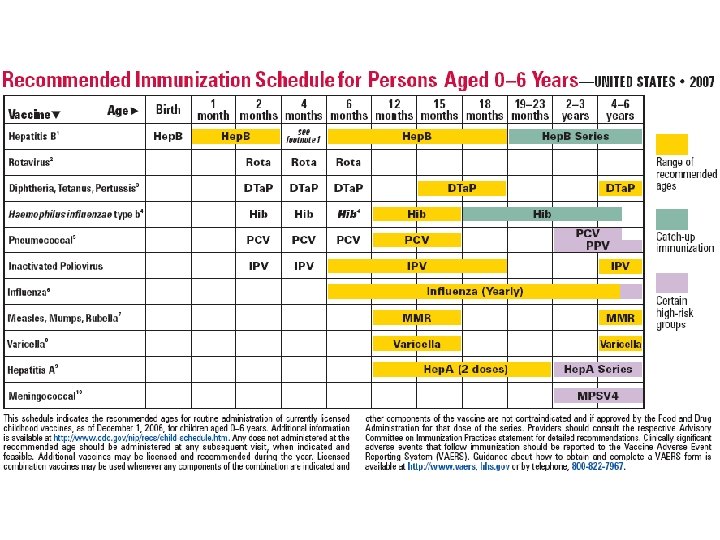
Number of Vaccines in the Routine Childhood and Adolescent Immunization Schedule 1985 1995 2006 Measles Rubella Mumps Diphtheria Tetanus Pertussis Polio Hib (infant) Hep. B Varicella Pneumococcal disease Influenza Meningococcal disease Hep. A Rotavirus HPV 7 10 16


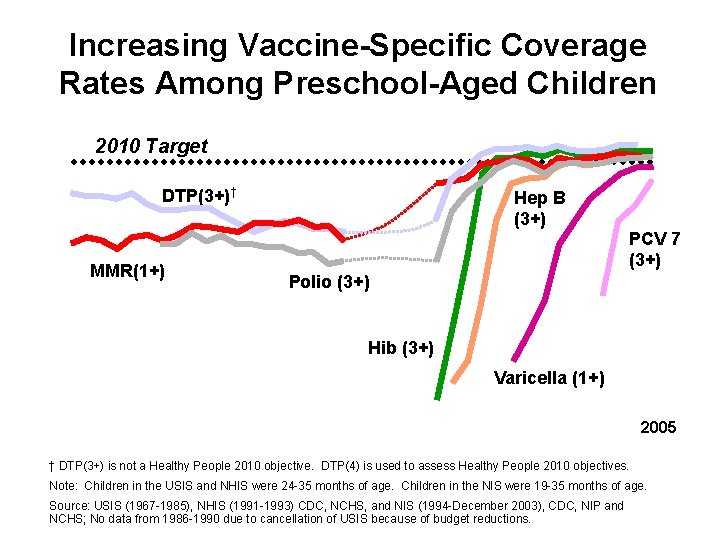
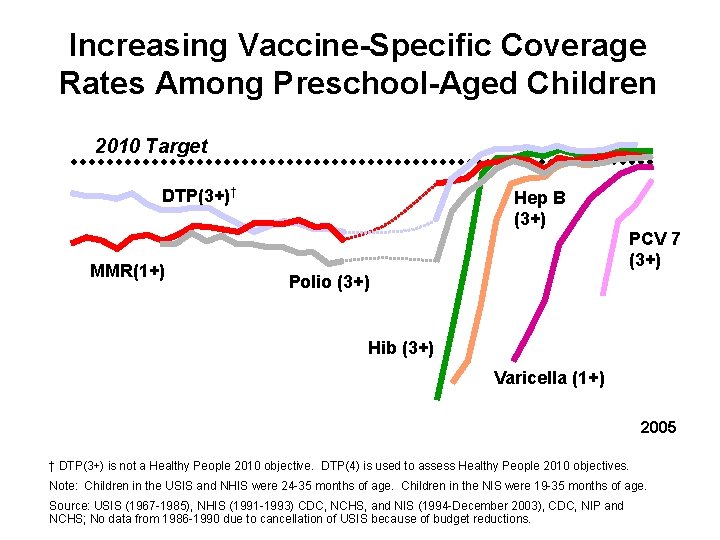
Increasing Vaccine-Specific Coverage Rates Among Preschool-Aged Children 2010 Target DTP(3+)† MMR(1+) Hep B (3+) PCV 7 (3+) Polio (3+) Hib (3+) Varicella (1+) 2005 † DTP(3+) is not a Healthy People 2010 objective. DTP(4) is used to assess Healthy People 2010 objectives. Note: Children in the USIS and NHIS were 24 -35 months of age. Children in the NIS were 19 -35 months of age. Source: USIS (1967 -1985), NHIS (1991 -1993) CDC, NCHS, and NIS (1994 -December 2003), CDC, NIP and NCHS; No data from 1986 -1990 due to cancellation of USIS because of budget reductions.

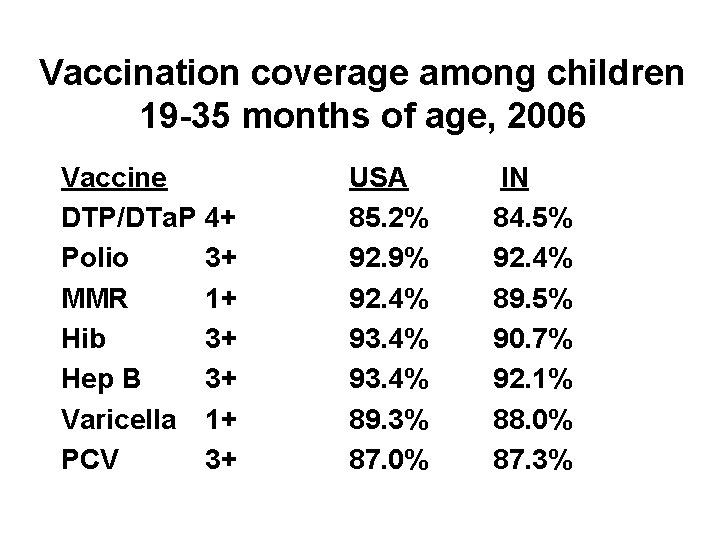
Vaccination coverage among children 19 -35 months of age, 2006 Vaccine DTP/DTa. P 4+ Polio 3+ MMR 1+ Hib 3+ Hep B 3+ Varicella 1+ PCV 3+ USA 85. 2% 92. 9% 92. 4% 93. 4% 89. 3% 87. 0% IN 84. 5% 92. 4% 89. 5% 90. 7% 92. 1% 88. 0% 87. 3%

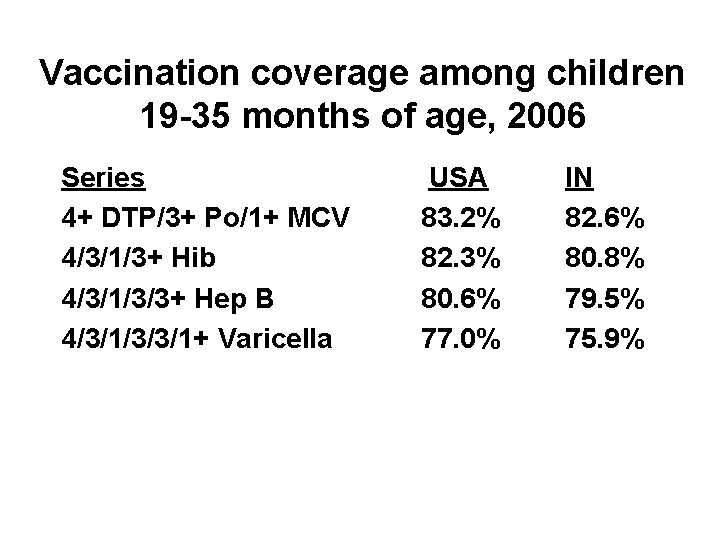
Vaccination coverage among children 19 -35 months of age, 2006 Series 4+ DTP/3+ Po/1+ MCV 4/3/1/3+ Hib 4/3/1/3/3+ Hep B 4/3/1/3/3/1+ Varicella USA 83. 2% 82. 3% 80. 6% 77. 0% IN 82. 6% 80. 8% 79. 5% 75. 9%

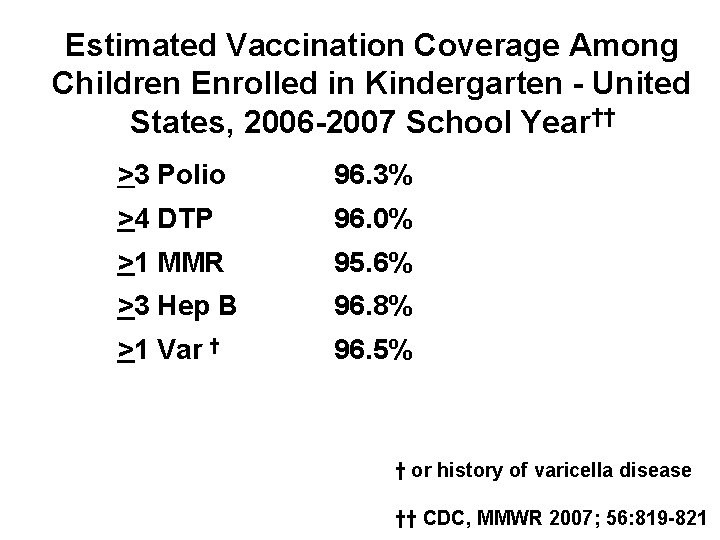
Estimated Vaccination Coverage Among Children Enrolled in Kindergarten - United States, 2006 -2007 School Year†† >3 Polio 96. 3% >4 DTP 96. 0% >1 MMR 95. 6% >3 Hep B 96. 8% >1 Var † 96. 5% † or history of varicella disease †† CDC, MMWR 2007; 56: 819 -821

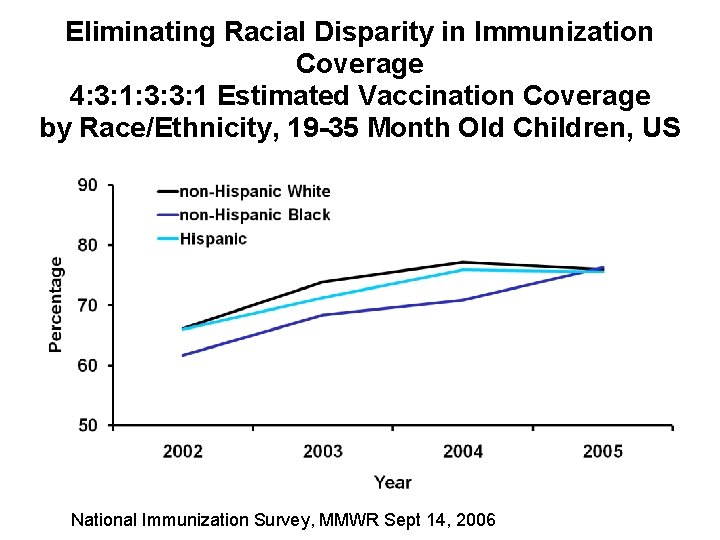
Eliminating Racial Disparity in Immunization Coverage 4: 3: 1: 3: 3: 1 Estimated Vaccination Coverage by Race/Ethnicity, 19 -35 Month Old Children, US National Immunization Survey, MMWR Sept 14, 2006

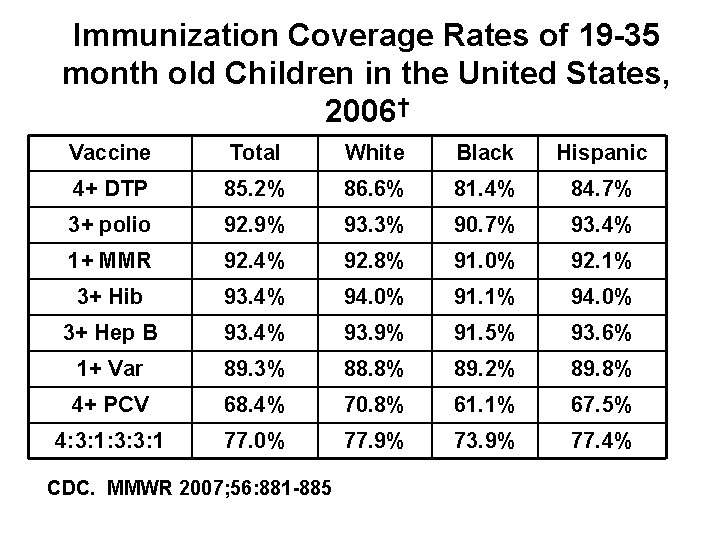
Immunization Coverage Rates of 19 -35 month old Children in the United States, 2006† Vaccine Total White Black Hispanic 4+ DTP 85. 2% 86. 6% 81. 4% 84. 7% 3+ polio 92. 9% 93. 3% 90. 7% 93. 4% 1+ MMR 92. 4% 92. 8% 91. 0% 92. 1% 3+ Hib 93. 4% 94. 0% 91. 1% 94. 0% 3+ Hep B 93. 4% 93. 9% 91. 5% 93. 6% 1+ Var 89. 3% 88. 8% 89. 2% 89. 8% 4+ PCV 68. 4% 70. 8% 61. 1% 67. 5% 4: 3: 1: 3: 3: 1 77. 0% 77. 9% 73. 9% 77. 4% CDC. MMWR 2007; 56: 881 -885

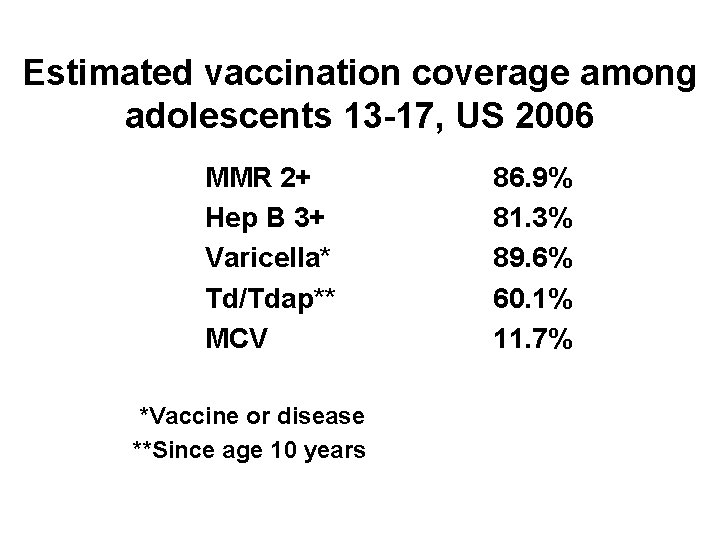
Estimated vaccination coverage among adolescents 13 -17, US 2006 MMR 2+ Hep B 3+ Varicella* Td/Tdap** MCV *Vaccine or disease **Since age 10 years 86. 9% 81. 3% 89. 6% 60. 1% 11. 7%

Types of costs in immunization • Vaccine purchase • Vaccine administration • Non-vaccine costs

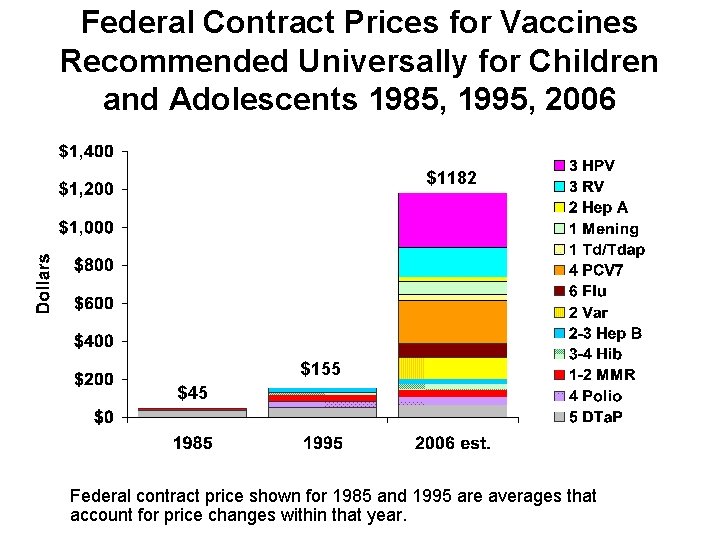
Federal Contract Prices for Vaccines Recommended Universally for Children and Adolescents 1985, 1995, 2006 $1182 $155 $45 Federal contract price shown for 1985 and 1995 are averages that account for price changes within that year.

Sources of financing childhood immunizations • Government – Federal – State/local • Insurance – Private – Public • Out-of-pocket

317 Immunization Program 317 grants support: • Purchase of vaccine for free administration at local health departments • Immunization delivery • Surveillance • Communication • Education

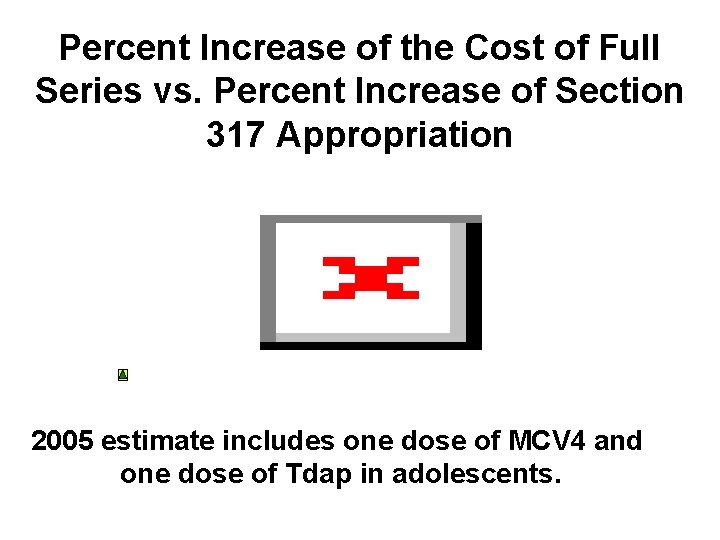
Percent Increase of the Cost of Full Series vs. Percent Increase of Section 317 Appropriation 2005 estimate includes one dose of MCV 4 and one dose of Tdap in adolescents.

Childhood Vaccine Doses Distributed by Funding Source Calendar Year 2005 Source: Vaccine manufacturers Biologics Surveillance Data 2005 Note: Does not include influenza vaccine

How Public Health Reaches Children through VFC • VFC program has 45, 000 provider sites – 75% of sites are private providers – 25% are public sector sites • Collectively, VFC providers vaccinate 90% of children – VFC vaccine for VFC-eligible children – Private purchase vaccine for other children • Improving VFC providers’ practices improves vaccinations for almost all children

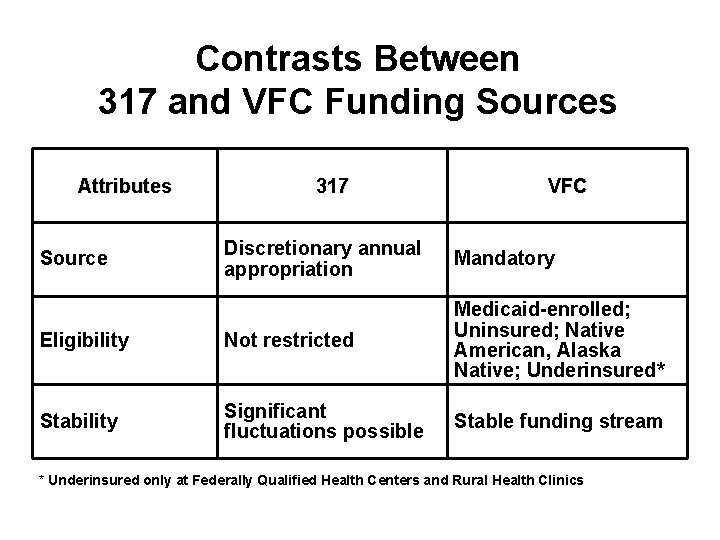
Contrasts Between 317 and VFC Funding Sources Attributes 317 VFC Discretionary annual appropriation Mandatory Eligibility Not restricted Medicaid-enrolled; Uninsured; Native American, Alaska Native; Underinsured* Stability Significant fluctuations possible Stable funding stream Source * Underinsured only at Federally Qualified Health Centers and Rural Health Clinics

VFC and Section 317 Vaccine Funding to Immunization Programs

Two-Tiered State Vaccination Policies at Local Health Departments • Traditionally, health department clinics vaccinated any child brought for vaccination • Underinsured children ineligible for VFC vaccine except at FQHCs and RHCs (~3, 000 clinics) – VFC designated FQHCs and RHCs as safety-net providers for underinsured children – State and 317 funding used for underinsured – Due to inadequate state/317 funding, many states cannot purchase vaccine for underinsured children • Result is a two-tiered policy – Government purchased vaccine not available to underinsured at health department clinics – Access to new vaccines for some based on insurance – Ethical tension for public health officials and providers

Two-Tiered States: 2005 • Invasive pneumococcal disease – 13 states did not purchase PCV 7 vaccine for underinsured children in health department clinics • Invasive meningococcal disease – 31 states did not purchase MCV 4 vaccine for underinsured children in health department clinics • These states do not have a public health department safety net to vaccinate children against these diseases

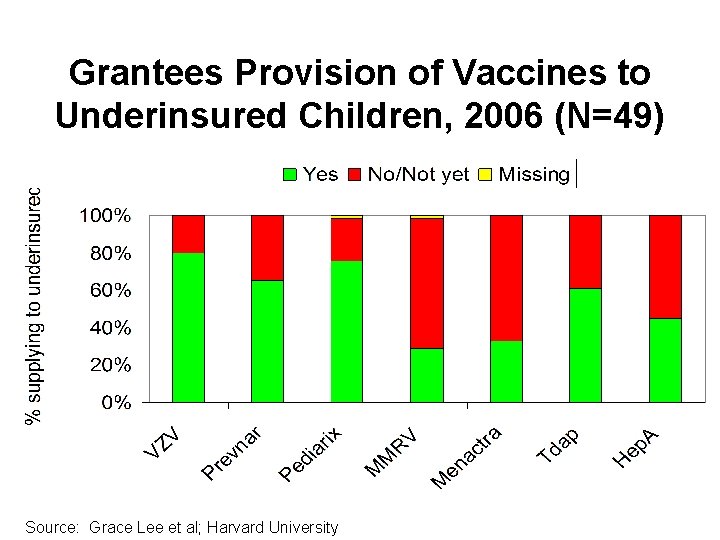
Grantees Provision of Vaccines to Underinsured Children, 2006 (N=49) Source: Grace Lee et al; Harvard University

Private insurance for childhood immunizations • Approximately 53% of children <5 in 2003 • Approximately 10% considered uninsured • Most insurers cover ACIP vaccines within approximately 3 months • Many require provider to purchase vaccine – up-front costs for inventory • Reimbursement may take some time and may not cover true cost of purchase

Public insurance for childhood immunizations • Medicare • Medicaid • CHIP – Medicaid enhancement – S-CHIP

Out-of-pocket expenses for childhood immunizations • • Primarily with underinsured children Providers may refer to health departments Exacerbated by costs of newer vaccines May be further exacerbated by efforts to make HPV mandatory without assuring that all children have access to vaccine in public sector

Inequities in vaccine purchase causes • Underinsurance • Inability of 317 and state/local funds to keep up with increasing vaccine costs • Burden on private providers to make large advance investments in purchasing new vaccines • Ethical dilemma at both state and provider levels

Inequities in vaccine purchase – some possible solutions • • Increase 317 appropriations Expand access to VFC for underinsured Allow access to VFC for S-CHIP Assure providers are fully reimbursed for purchase costs • Allow delayed payments to vaccine manufacturers/distributors

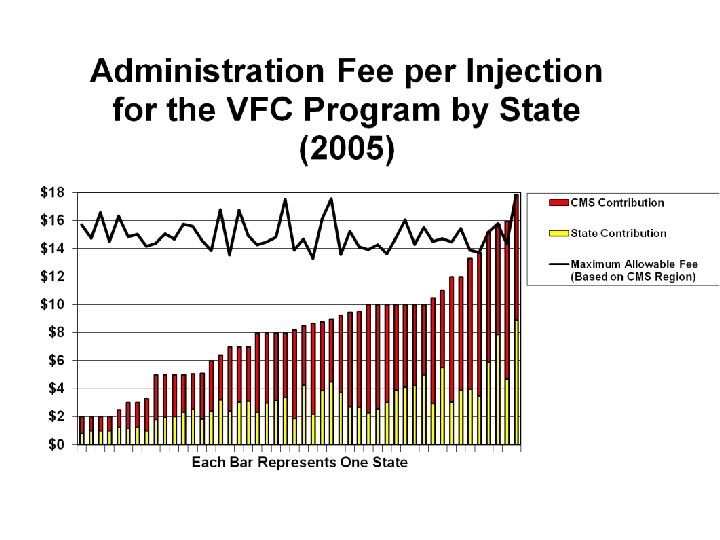
Vaccine administration • Studies indicate that it costs approximately $18 -25/injection to administer vaccines • VFC does not reimburse for vaccine administration • Widely varying rates of reimbursement from Medicaid and private insurers


Inequities in vaccine administration reimbursement • Some possible solutions – Reimbursement from VFC – Minimum/recommended reimbursement rate from CMS – Negotiation with private insurers

Non-vaccine costs • Include costs of – Acquiring vaccine – Storing vaccine – Handling vaccine – Loss of vaccines – Infrastructure – Insurance

Inequities in non-vaccine costs causes • 317 can support HD costs • VFC supports infrastructure and program costs; administration fees, in theory, include non-vaccine costs – However, Medicaid establishes reimbursement rate • Private insurers typically do not include non-vaccine costs in calculating reimbursement rates

Inequities in non-vaccine costs – some possible solutions • CMS establishes minimum or recommended reimbursement rate • Private insurers encouraged to include non-vaccine costs in reimbursement calculations

What is being done • AAP Task Force on Immunization • NVAC Financing Work Group • IDSA workgroup on adult and adolescent immunization • AMA/AAP/IDSA National Immunization Congress

Recommendations from Immunization Congress - 1 • Work with FQHCs to delegate authority to public health clinics to serve underinsured through VFC • Obtain data on cost of delivering vaccines in private practice setting and use data to educate payers and advocate for better payment • Work with manufacturers/distributors to obtain more favorable terms for payments for vaccine inventories

Recommendations from Immunization Congress - 2 • Better define components of CPT codes for immunization administration • Examine potential role of tax credits for insurers or employers in eliminating underinsurance • Create working group to explore possibility of federal vaccine purchase or funding mechanism

Recommendations from Immunization Congress - 3 • Obtain from CMS the data that led to current Medicare administration fee and use data to advocate at state level for enhanced payment • Collect data on true cost of obtaining/delivering combination vaccines as opposed to individual vaccines • Disseminate information on best business practices to minimize vaccine and administration costs

Increasing Vaccine-Specific Coverage Rates Among Preschool-Aged Children 2010 Target DTP(3+)† MMR(1+) Hep B (3+) PCV 7 (3+) Polio (3+) Hib (3+) Varicella (1+) 2005 † DTP(3+) is not a Healthy People 2010 objective. DTP(4) is used to assess Healthy People 2010 objectives. Note: Children in the USIS and NHIS were 24 -35 months of age. Children in the NIS were 19 -35 months of age. Source: USIS (1967 -1985), NHIS (1991 -1993) CDC, NCHS, and NIS (1994 -December 2003), CDC, NIP and NCHS; No data from 1986 -1990 due to cancellation of USIS because of budget reductions.

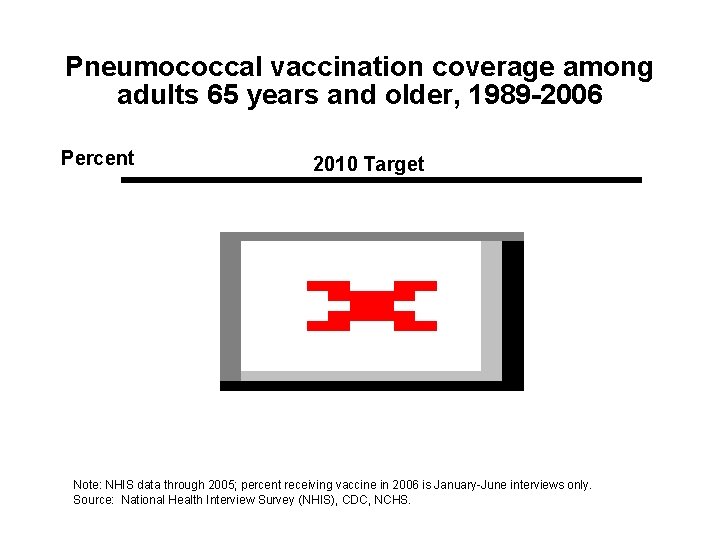
Pneumococcal vaccination coverage among adults 65 years and older, 1989 -2006 Percent 2010 Target Note: NHIS data through 2005; percent receiving vaccine in 2006 is January-June interviews only. Source: National Health Interview Survey (NHIS), CDC, NCHS.

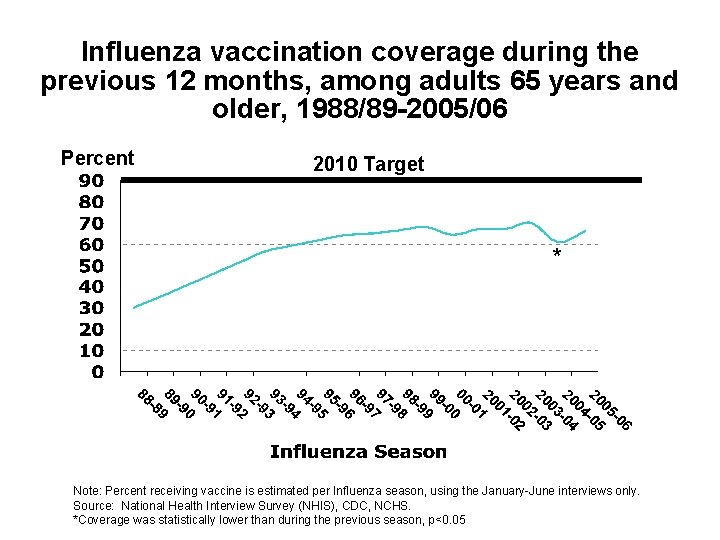
Influenza vaccination coverage during the previous 12 months, among adults 65 years and older, 1988/89 -2005/06 Percent 2010 Target * Note: Percent receiving vaccine is estimated per Influenza season, using the January-June interviews only. Source: National Health Interview Survey (NHIS), CDC, NCHS. *Coverage was statistically lower than during the previous season, p<0. 05

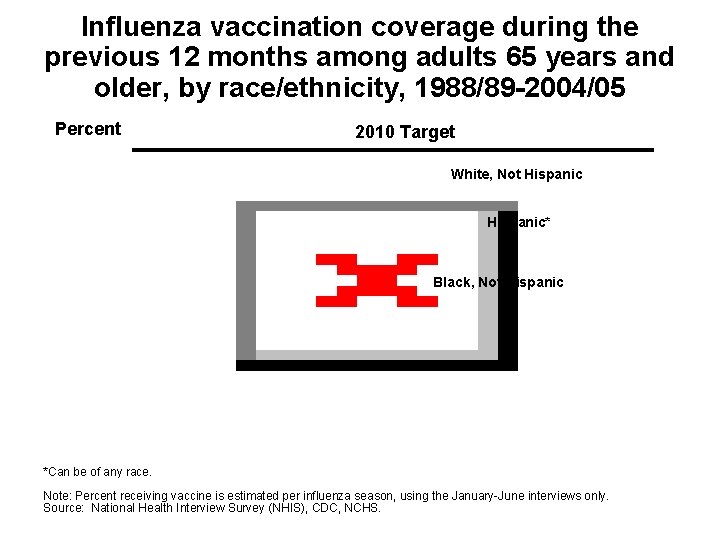
Influenza vaccination coverage during the previous 12 months among adults 65 years and older, by race/ethnicity, 1988/89 -2004/05 Percent 2010 Target White, Not Hispanic* Black, Not Hispanic *Can be of any race. Note: Percent receiving vaccine is estimated per influenza season, using the January-June interviews only. Source: National Health Interview Survey (NHIS), CDC, NCHS.

Differentiating characteristics of childhood and adult immunization • Delivery system infrastructure • Financing mechanisms • Highly effective vaccines preventing recognizable diseases • Patient/parent/societal expectations • Provider attitudes • Leadership of state/local health departments and CDC

Delivery system infrastructure • Both children and adults receive most immunizations in private sector • Health departments a safety net for children, not so much for adults • VFC provides strong ties between health departments and private providers; nothing comparable for adults

Financing mechanisms • Uninsured children have entitlement to free vaccine through Vaccines for Children (VFC); cost not really a barrier except to underinsured • Adults >65 have entitlement through Medicare • No comparable mechanism for the 16% of adults <65 who are uninsured – cost is a barrier • 317 funds can be used for children and adults but children have gotten priority

Patient/parent/societal expectations • School immunization requirements for children • National vaccine injury compensation program for children • Nothing comparable for adults

Provider attitudes • Physician is the most trusted source of information about immunization • Immunization/well visits a major part of clinical care for children; less so for clinical care of adults • Pneumococcal and influenza vaccines not as effective as childhood vaccines; may be less perceived benefit to provider

Leadership of state/local health departments and CDC • Childhood immunization program structure in each state health department; few states have adult immunization program structure • National Immunization Survey for children; BRFSS for adults • States respond to low coverage in children; not so much to low coverage in adults

NVAC 2005 recommendations • Expanded funding through Section 317 to support adolescent and adult immunization programs • Promotion of “first-dollar” insurance coverage • Assurance of adequate reimbursement for administration of vaccines • Expanded discussion about the need, desirability, and feasibility of a variety of approaches to ensure that adults have access to vaccines, even if they do not have insurance

Partnership for Prevention 2005 recommendations • Purchase and distribute influenza vaccine for uninsured adults • Ensure first-dollar coverage for influenza and pneumococcal vaccines in the Federal Employee Health Benefit Program • Expand Section 317 of the Public Health Service Act to address adult immunization needs • Launch a national campaign to educate Americans about the value of adult immunizations

IDSA 2007 working principles • • Increase demand Strengthen capacity to deliver Expand provision of vaccines in insurance Promote immunization as a measure of health care quality • Monitor and improve performance of the vaccine delivery and safety monitoring systems • Assure adequate support for research

What are the minimum actions we should take? • Increase 317 appropriations with an earmark for adult immunizations • Establish an infrastructure for promoting/coordinating adult immunizations • Ensure adequate reimbursement for vaccine administration • Establish a culture of immunization in those who provide care to adults

Conclusions • Private-public partnership has improved childhood immunization rates – Increasing 317 appropriations – Implementation of VFC • Increasing number and costs of vaccines have put strains on system resulting in inequities • Unless resolved, these inequities may undermine our current successes • Dealing with adolescents and adults adds complexity