Chemotherapy for endometrial cancer Amelia Jernigan MD Assistant












- Slides: 32

+ Chemotherapy for endometrial cancer Amelia Jernigan, MD Assistant Professor of Gynecologic Oncology LSU-New Orleans November 11, 2016

+ Objectives n To review the types of endometrial cancers and general approaches to treatment n Type II (grade III, UPSC, Carcinosarcoma, clear cell) n Review the role of chemotherapy in the treatment of endometrial cancer in the following settings n Adjuvant n Recurrent/metastatic n Neoadjuvant n To discuss emerging therapeutic approaches & exciting developments

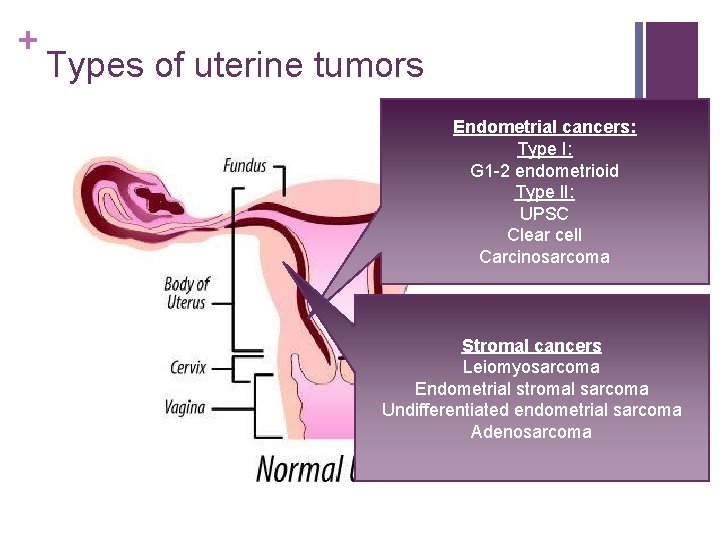
+ Types of uterine tumors Endometrial cancers: Type I: G 1 -2 endometrioid Type II: UPSC Clear cell Carcinosarcoma Stromal cancers Leiomyosarcoma Endometrial stromal sarcoma Undifferentiated endometrial sarcoma Adenosarcoma

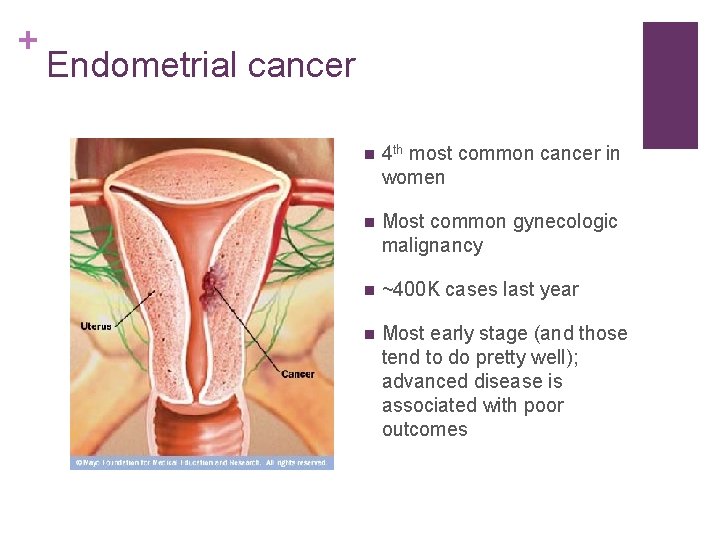
+ Endometrial cancer n 4 th most common cancer in women n Most common gynecologic malignancy n ~400 K cases last year n Most early stage (and those tend to do pretty well); advanced disease is associated with poor outcomes

+ Staging (out with the old & in with the new – but ya gotta know both!)

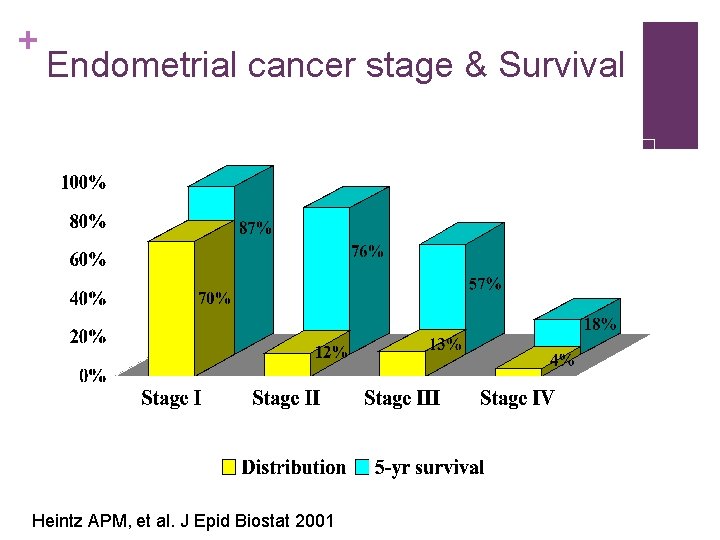
+ Endometrial cancer stage & Survival Heintz APM, et al. J Epid Biostat 2001

+ Treatment for early stage disease n Surgery n TAH n BSO (usually) n Lymph node assessment n Strategies n Fertility preservation option: n No LND n D&C q 3 months n Selective LND based on intraoperative findings n n LND for everyone Progesterone (megace, medroxyprogesterone, Mirena IUD) n Sentinel lymph node biopsy RISK OF PELVIC LN METASTASIS Invasion Grade 1 Grade 2 Grade 3 Endometrium Inner 1/3 Middle 1/3 Outer 1/3 0% 3% 0% 11% 3% 5% 9% 19% 0% 9% 5% 34% Creaseman WT Cancer 1987; Mariani Gyn Onc 2008

+ Adjuvant therapy for early stage disease – high intermediate risk? PORTEC 1 n n Included: Stage I EC: G 1 -2 >50%, G 2 -3 <50% Excluded: serous, clear cell n TAH, BSO, no LND n No adj t vs RT n 8 year LRR 4% vs 14% (p<0. 001), OS no difference, increased morbidity n HIR: >60, G 3, Outer half GOG-99 n Included: Stage IB, IC, IIA (occult), IIB (occult). n Excluded: serous, clear cell n TAH/BSO Lymph node sampling n No adj tx vs RT n HIR: RF – G 2 -3, LVI+, outer 1/3 n Any age – all 3 risk factors n 50 -69 – 2 risk factors n >=70 – any risk factor n Risk of recurrence was lower in the HIR group; did not set out to or show difference in OS.

+ Vaginal cuff brachytherapy n PORTEC 2: n Stage IBG 3, ICG 1 -2 >60 yoa, IIAG 1 -2, G 3 >1/2 invasion n EBRT vs Brachytherapy n No difference in vaginal recurrence or distant recurrence. More pelvic recurrence (3. 5% vs 0. 6%) n No difference in OS (but much death is due to intercurrent diseaes)

+ The role of chemotherapy n Hogberg trial n Two RCT: NSGO-EC 9501/EORTC-55991 & MANGO-ILIADE-III n Stage I-III EC, no residual tumor, and high risk disease n Randomized: RT with or without sequential chemotherapy n ~ 36% reduction in risk of relapse or death (p = 0. 07 combined, sig for NSGO but not MANGO)

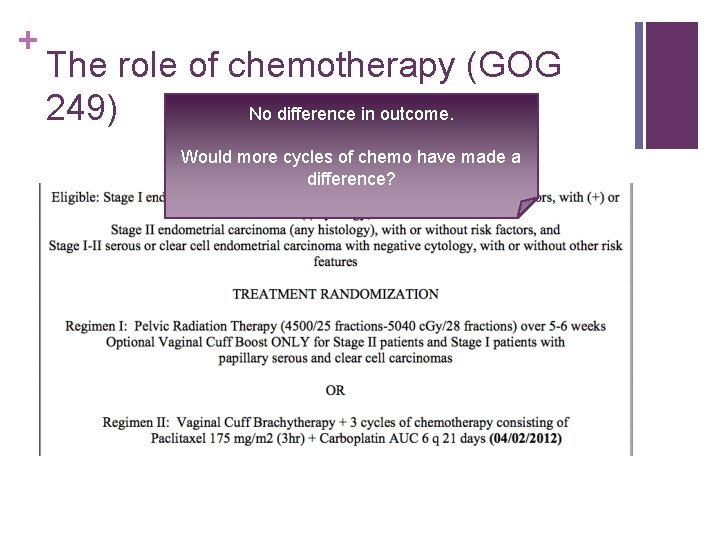
+ The role of chemotherapy (GOG 249) No difference in outcome. Would more cycles of chemo have made a difference?

+ What about chemo. RT and more chemo? – RTOG 9708 n Phase II clinical trial n High risk EC (g 2 -3 with >50% MI, cervical stromal invasion or pelvic confined extrauterine disease) n Cis 50 mg/msq D 1 & 28 during RT (45 Gy) to pelvis followed by vaginal brachyteherapy and then cis 50 mg/msq and paclitaxel 175 mg/msq every 4 weeks for 4 cycles n At 4 years: pelvic, regional and distant reucurence rates – 2%, 2% and 19% n 4 year OS and DFS: 85% and 81% n 4 year OS and DFS for stage III patients: 77 % 72%; no recurrences for stage IC-IIA ***

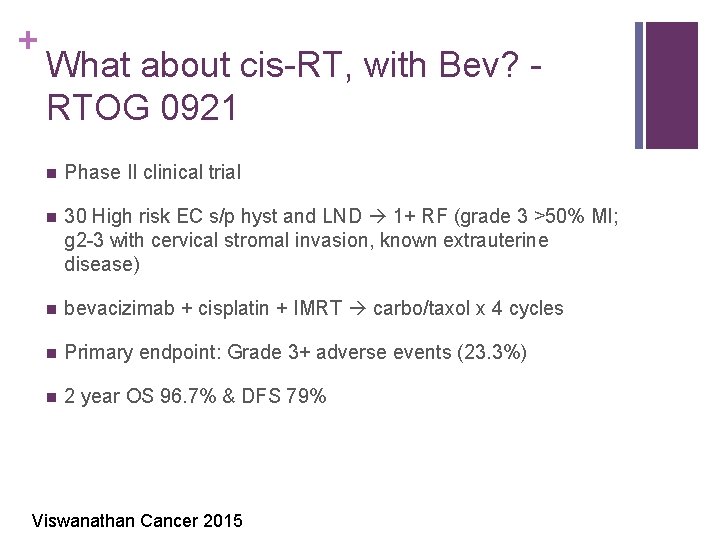
+ What about cis-RT, with Bev? RTOG 0921 n Phase II clinical trial n 30 High risk EC s/p hyst and LND 1+ RF (grade 3 >50% MI; g 2 -3 with cervical stromal invasion, known extrauterine disease) n bevacizimab + cisplatin + IMRT carbo/taxol x 4 cycles n Primary endpoint: Grade 3+ adverse events (23. 3%) n 2 year OS 96. 7% & DFS 79% Viswanathan Cancer 2015

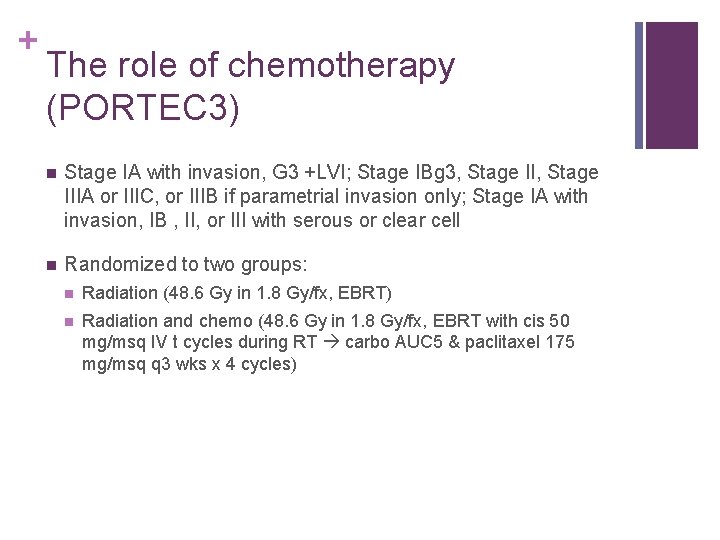
+ The role of chemotherapy (PORTEC 3) n Stage IA with invasion, G 3 +LVI; Stage IBg 3, Stage IIIA or IIIC, or IIIB if parametrial invasion only; Stage IA with invasion, IB , II, or III with serous or clear cell n Randomized to two groups: n Radiation (48. 6 Gy in 1. 8 Gy/fx, EBRT) n Radiation and chemo (48. 6 Gy in 1. 8 Gy/fx, EBRT with cis 50 mg/msq IV t cycles during RT carbo AUC 5 & paclitaxel 175 mg/msq q 3 wks x 4 cycles)

+ Treatment for advanced stage disease n Surgery: n Debulking gross disease when you can n Chemotherapy has trumped radiation

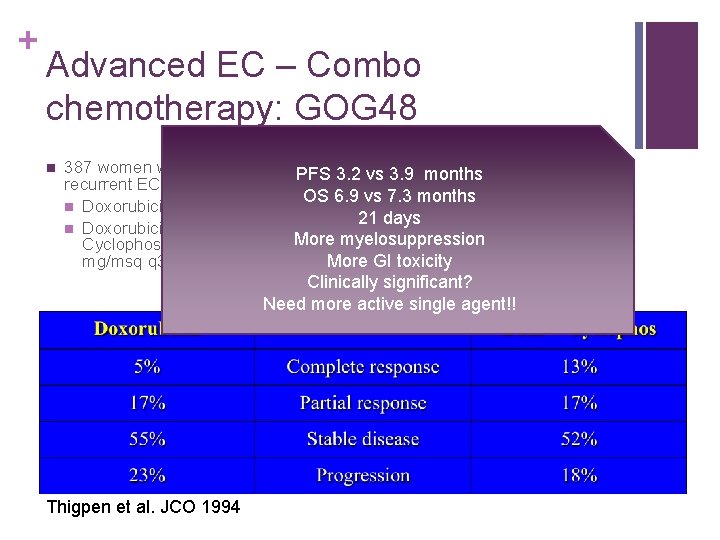
+ Advanced EC – Combo chemotherapy: GOG 48 n 387 women with advanced or PFS 3. 2 n vs Conclusion: Doxorubicin + 3. 9 months recurrent EC RCT cyclophosphamide appears to OS 6. 9 vs 7. 3 amonths offer small advantage over n Doxorubicin 60 mg/msq q 3 wks alone in management 21 doxorubicin days n Doxorubicin 60 mg/msq + of EC at the expense of more More myelosuppression Cyclophosphamide 500 frequent myelosuppression mg/msq q 3 wks More and GI toxicity Clinically significant? Need more active single agent!! Thigpen et al. JCO 1994

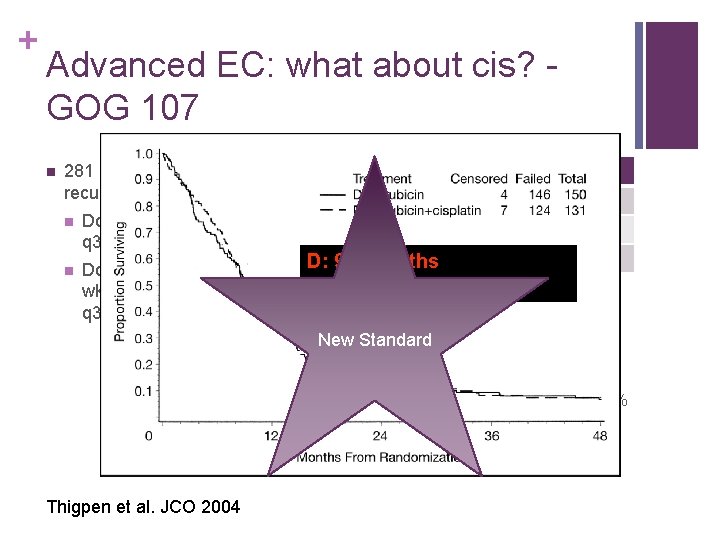
+ Advanced EC: what about cis? GOG 107 n 281 patients with advanced or recurrent EC randomized n n Doxorubicin 60 mg/msq q 3 wks D Response Cis+D 8% Complete 19% 17% Partial 23% D: 3. 8 months 75% None 58% D: 9. 2 5. 7 months Doxorubicin 60 mg/msq q 3 D+Cis: months wks and cisplatin 50 mg/msq. D + Cisp: 9. 0 months q 3 wks n Combination arm with more n G 4 leukopenia (28% vs 7. 4%) n G 3&4 anemia (19% & 5. 4% vs 4% & 0%) n G 3&4 Thrombocytopenia (8. 5% & 5. 4% vs 1. 3% & 0. 7%) n Nausea & vomiting New Standard Thigpen et al. JCO 2004

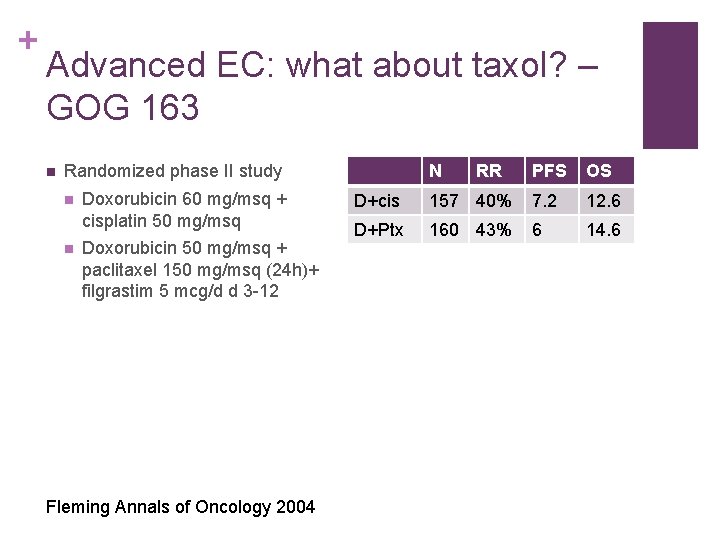
+ Advanced EC: what about taxol? – GOG 163 n N Randomized phase II study n n Doxorubicin 60 mg/msq + cisplatin 50 mg/msq Doxorubicin 50 mg/msq + paclitaxel 150 mg/msq (24 h)+ filgrastim 5 mcg/d d 3 -12 Fleming Annals of Oncology 2004 RR PFS OS D+cis 157 40% 7. 2 12. 6 D+Ptx 160 43% 6 14. 6

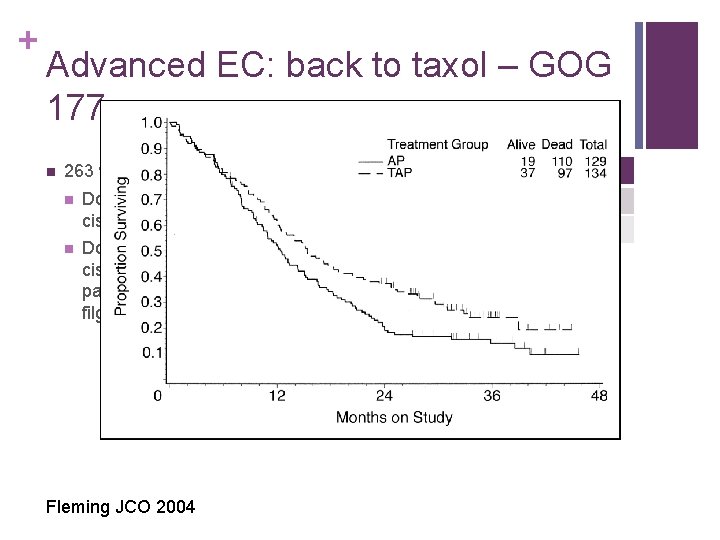
+ Advanced EC: back to taxol – GOG 177 n RR PFS OS AP 34% 5. 3 12. 3 TAP 57% 8. 3 15. 3 263 women n n Doxorubicin 60 mg/msq + cisplatin 50 mg/msq Doxorubicin 50 mg/msq, cisplatin 50 mg/msq, pacitaxel 160 mg/msq, filgrastim Fleming JCO 2004

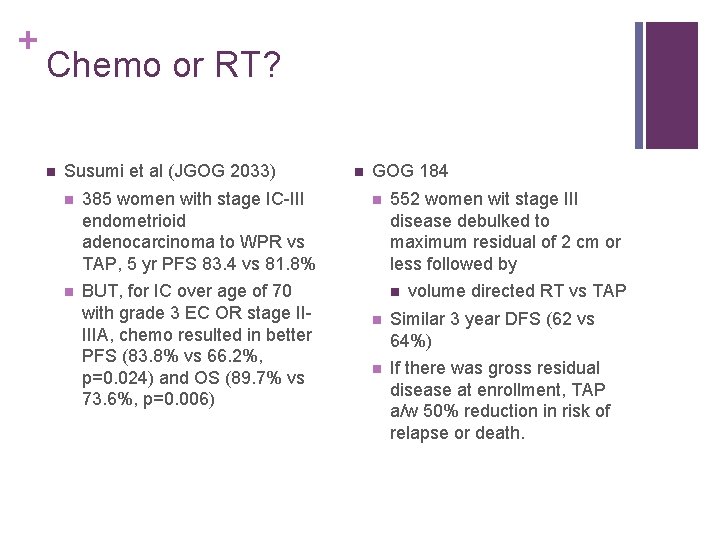
+ Chemo or RT? n Susumi et al (JGOG 2033) n 385 women with stage IC-III endometrioid adenocarcinoma to WPR vs TAP, 5 yr PFS 83. 4 vs 81. 8% n BUT, for IC over age of 70 with grade 3 EC OR stage IIIIIA, chemo resulted in better PFS (83. 8% vs 66. 2%, p=0. 024) and OS (89. 7% vs 73. 6%, p=0. 006) n GOG 184 n 552 women wit stage III disease debulked to maximum residual of 2 cm or less followed by n volume directed RT vs TAP n Similar 3 year DFS (62 vs 64%) n If there was gross residual disease at enrollment, TAP a/w 50% reduction in risk of relapse or death.

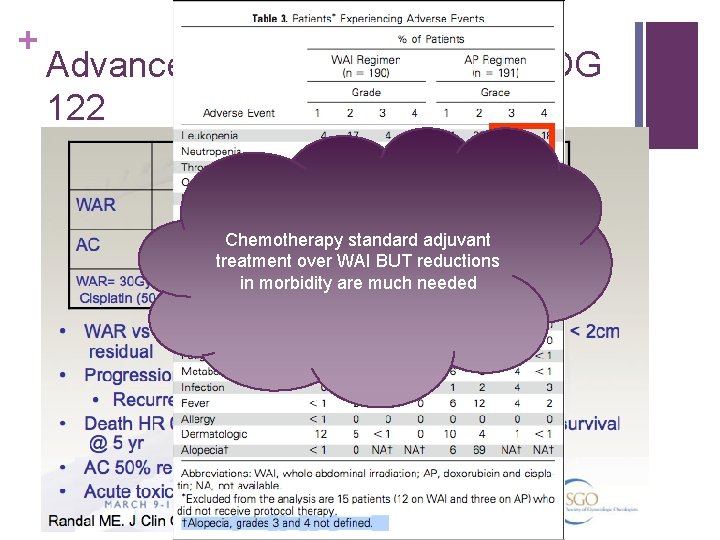
+ Advanced EC – WAI vs AP – GOG 122 Chemotherapy standard adjuvant treatment over WAI BUT reductions in morbidity are much needed

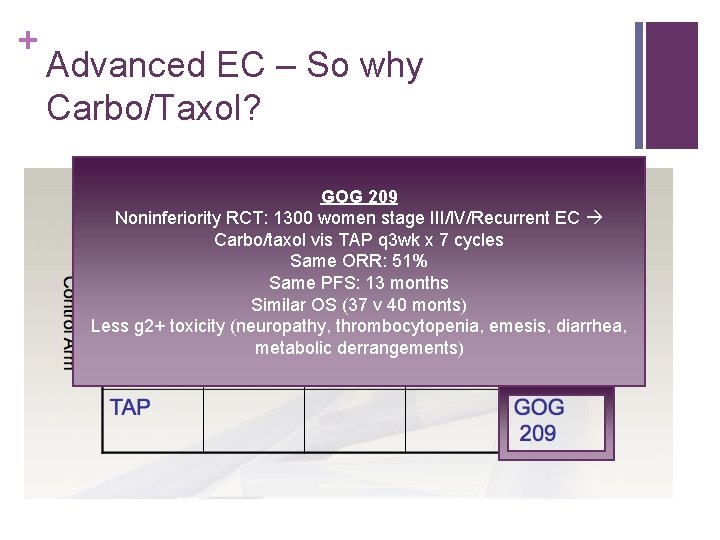
+ Advanced EC – So why Carbo/Taxol? GOG 209 Noninferiority RCT: 1300 women stage III/IV/Recurrent EC Carbo/taxol vis TAP q 3 wk x 7 cycles Same ORR: 51% Same PFS: 13 months Similar OS (37 v 40 monts) Less g 2+ toxicity (neuropathy, thrombocytopenia, emesis, diarrhea, metabolic derrangements)

+ What about sequencing? n Retrospective data suggests that sandwich therapy may result in improve outcomes (OS, PFS) compared to chemo/radiation or radiation/chemo – but this has been difficult to reproduce Secord Gyn Onc 2009

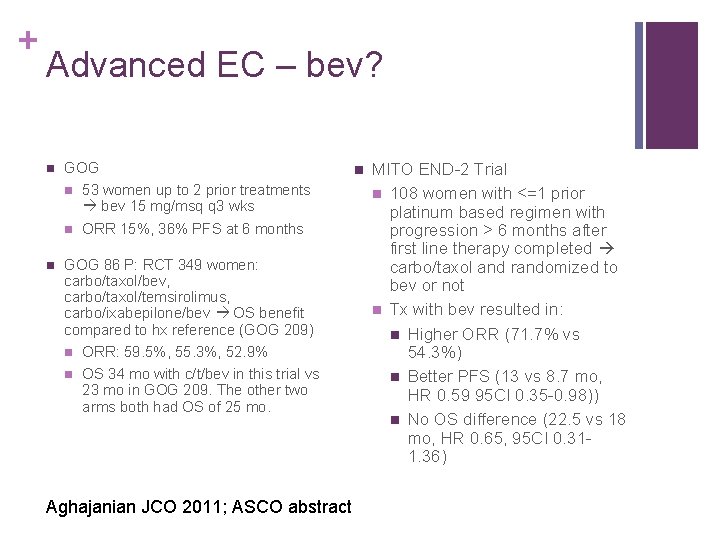
+ Advanced EC – bev? n GOG n 53 women up to 2 prior treatments bev 15 mg/msq q 3 wks n ORR 15%, 36% PFS at 6 months n GOG 86 P: RCT 349 women: carbo/taxol/bev, carbo/taxol/temsirolimus, carbo/ixabepilone/bev OS benefit compared to hx reference (GOG 209) n ORR: 59. 5%, 55. 3%, 52. 9% n OS 34 mo with c/t/bev in this trial vs 23 mo in GOG 209. The other two arms both had OS of 25 mo. Aghajanian JCO 2011; ASCO abstract n MITO END-2 Trial n 108 women with <=1 prior platinum based regimen with progression > 6 months after first line therapy completed carbo/taxol and randomized to bev or not n Tx with bev resulted in: n Higher ORR (71. 7% vs 54. 3%) n Better PFS (13 vs 8. 7 mo, HR 0. 59 95 CI 0. 35 -0. 98)) n No OS difference (22. 5 vs 18 mo, HR 0. 65, 95 CI 0. 311. 36)

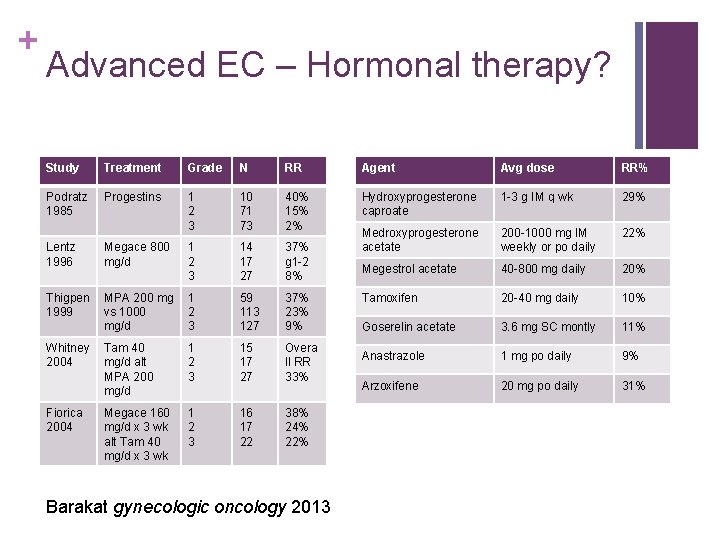
+ Advanced EC – Hormonal therapy? Study Treatment Grade N RR Agent Avg dose RR% Podratz 1985 Progestins 1 2 3 10 71 73 40% 15% 2% Hydroxyprogesterone caproate 1 -3 g IM q wk 29% Megace 800 mg/d 1 2 3 14 17 27 37% g 1 -2 8% Medroxyprogesterone acetate 200 -1000 mg IM weekly or po daily 22% Lentz 1996 Megestrol acetate 40 -800 mg daily 20% Thigpen 1999 MPA 200 mg vs 1000 mg/d 1 2 3 59 113 127 37% 23% 9% Tamoxifen 20 -40 mg daily 10% Goserelin acetate 3. 6 mg SC montly 11% Tam 40 mg/d alt MPA 200 mg/d 1 2 3 15 17 27 Overa ll RR 33% Anastrazole 1 mg po daily 9% Arzoxifene 20 mg po daily 31% Megace 160 mg/d x 3 wk alt Tam 40 mg/d x 3 wk 1 2 3 16 17 22 38% 24% 22% Whitney 2004 Fiorica 2004 Barakat gynecologic oncology 2013

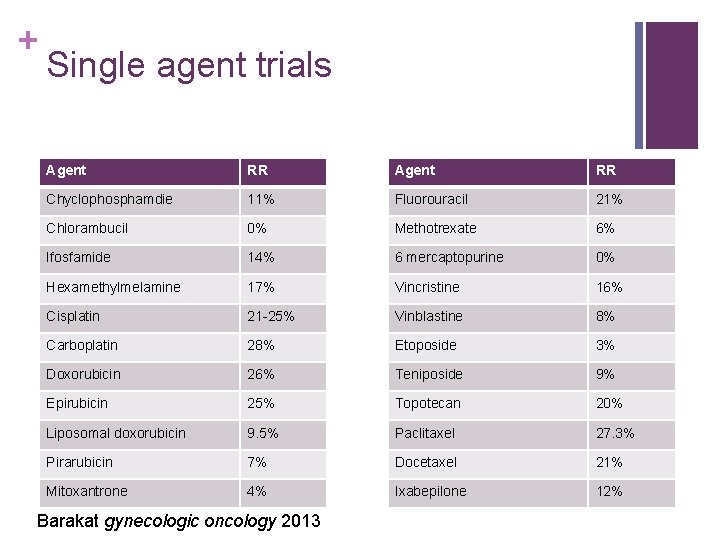
+ Single agent trials Agent RR Chyclophosphamdie 11% Fluorouracil 21% Chlorambucil 0% Methotrexate 6% Ifosfamide 14% 6 mercaptopurine 0% Hexamethylmelamine 17% Vincristine 16% Cisplatin 21 -25% Vinblastine 8% Carboplatin 28% Etoposide 3% Doxorubicin 26% Teniposide 9% Epirubicin 25% Topotecan 20% Liposomal doxorubicin 9. 5% Paclitaxel 27. 3% Pirarubicin 7% Docetaxel 21% Mitoxantrone 4% Ixabepilone 12% Barakat gynecologic oncology 2013

+ Special situations UPSC n n Fader – multiinstitutional, retrospective study of stage I UPSC s/p Staging – Obs vs RT vs Plat/tax chemo & RT But (p=0. 013) what about Chemotherapy fewer recurrences noninvasive, stage IA, n CT +/- RT: 11. 2% Recur completely staged n RT: 25% recur (NS vs CT +/-RT) patients? ? ? *** may not need n Obs: 30. 3% recur chemo*** Fader Cancer 2009; Mahdi Gyn Onc 2015

+ Special situations - UPSC n Serous cancers n Neoadjuvant response rates to carbo and taxol of 60 -70% n Surgical debulking and staging should be routinely performed when feasible n Platinum based chemotherapy should be considered in all patients n n …. ? Confined to a polyp and fully staged? ? Radiation?

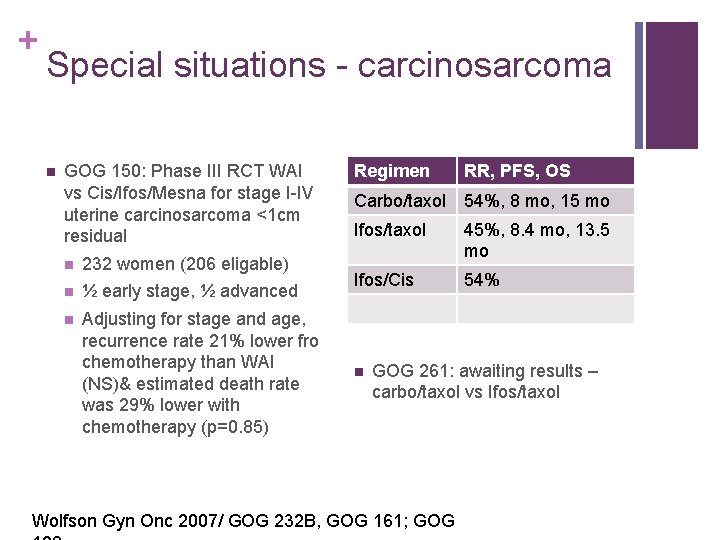
+ Special situations - carcinosarcoma n GOG 150: Phase III RCT WAI vs Cis/Ifos/Mesna for stage I-IV uterine carcinosarcoma <1 cm residual n 232 women (206 eligable) n ½ early stage, ½ advanced n Adjusting for stage and age, recurrence rate 21% lower fro chemotherapy than WAI (NS)& estimated death rate was 29% lower with chemotherapy (p=0. 85) Regimen RR, PFS, OS Carbo/taxol 54%, 8 mo, 15 mo Ifos/taxol 45%, 8. 4 mo, 13. 5 mo Ifos/Cis 54% n GOG 261: awaiting results – carbo/taxol vs Ifos/taxol Wolfson Gyn Onc 2007/ GOG 232 B, GOG 161; GOG

+ Special circumstances – uterine carcinosarcoma n Topotecan may have promise in the recurrent setting n 51 women with recurrent or progressive Uterine carcinosarcoma n Topotecan 1. 5 mg/msq IV x 5 days every 3 weeks until progression or intolerance n 3 (6%) died after first tx n 5 complete responses (10%) n 13 stable disease (27%) Miller, Gyn Onc 2005

+ Special circumstances n Recurrence limited to the vagina n If no prior RT Most vaginal recurrences are salvageable, on long term (~15 year) follow up of PORTEC 1: n If prior RT n n If candidate, surgery (exenteration) n If not candidate for surgery Tailored RT or medical therapy Creutzberg IJROBP 2011 n Survival rates after vaginal recurrence were 70% for no tx and 38% for EBRT at 5 years & 51% vs 25% at 1 years.

+ Thanks!
 Endometrial cancer
Endometrial cancer Endometrial cancer
Endometrial cancer Dr christopher jernigan
Dr christopher jernigan Dennis jernigan you are my hero
Dennis jernigan you are my hero Kolopskopi
Kolopskopi General principles of chemotherapy
General principles of chemotherapy 4ac 4t chemotherapy
4ac 4t chemotherapy Bsa calculation formula for chemotherapy
Bsa calculation formula for chemotherapy Chemotherapy
Chemotherapy Chemotherapy coding cheat sheet
Chemotherapy coding cheat sheet Chemotherapy
Chemotherapy Principles of chemotherapy
Principles of chemotherapy Caf framework
Caf framework Pablo cubillos
Pablo cubillos Spotting differential diagnosis
Spotting differential diagnosis Uterus diagram
Uterus diagram Endometrial histology menstrual cycle
Endometrial histology menstrual cycle Endometrial cells
Endometrial cells Hipotlamo
Hipotlamo Modified shirodkar cerclage
Modified shirodkar cerclage Endometrial polyp gross
Endometrial polyp gross It projektov�� mana����r
It projektov�� mana����r Grosor endometrial normal
Grosor endometrial normal What is this organ
What is this organ Endometrial adenocarcinoma
Endometrial adenocarcinoma Innocent witches amelia
Innocent witches amelia What inference can be made about amelia earhart?
What inference can be made about amelia earhart? Amelia earhart paragraph
Amelia earhart paragraph Pygmy clowns
Pygmy clowns July 2 1937 amelia earhart
July 2 1937 amelia earhart Amelia earhart solo flight across the atlantic
Amelia earhart solo flight across the atlantic Amelia earhart presentation
Amelia earhart presentation Amelia holt
Amelia holt