Chemistry Vocabulary Part 1 Matter Anything that has

Chemistry Vocabulary: Part 1

Matter � Anything that has mass and takes up space � Made up of tiny particles called atoms Atoms: smallest particle of matter

Atoms �Smallest unit of an element that maintains the properties of that element �Can not be broken down into anything smaller �Scientists used to think they couldn’t be split, but we now know that was incorrect
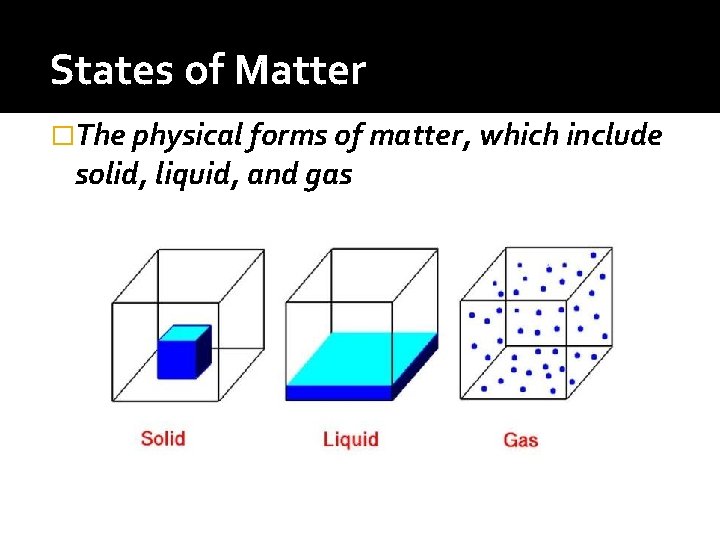
States of Matter �The physical forms of matter, which include solid, liquid, and gas
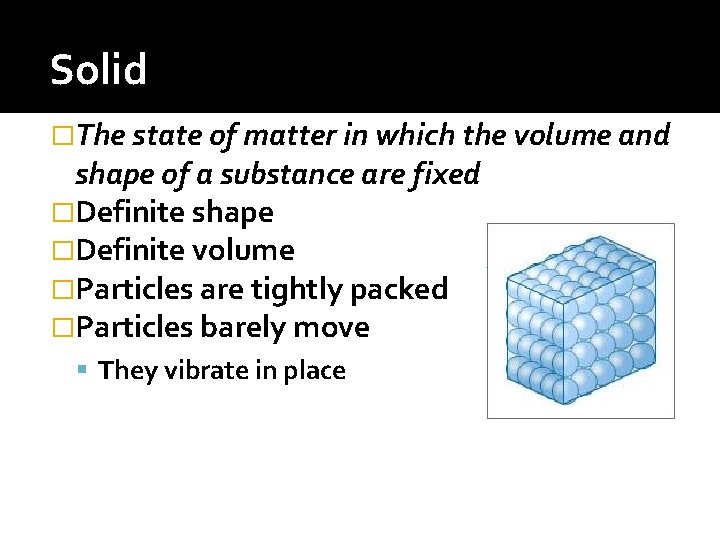
Solid �The state of matter in which the volume and shape of a substance are fixed �Definite shape �Definite volume �Particles are tightly packed �Particles barely move They vibrate in place
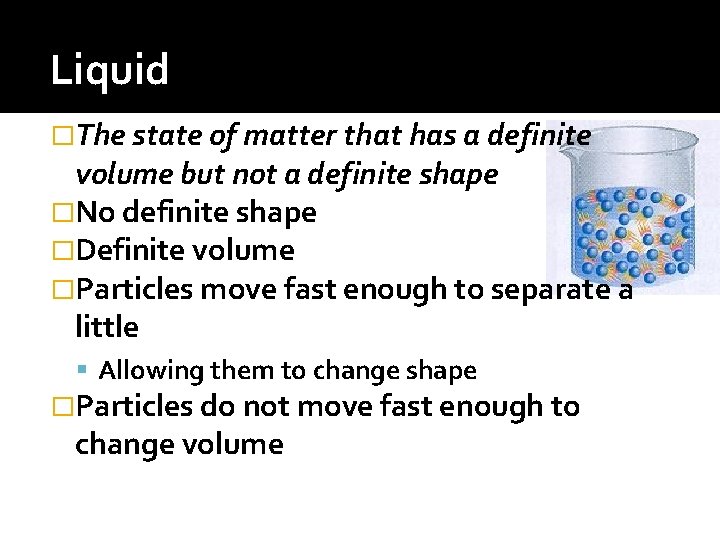
Liquid �The state of matter that has a definite volume but not a definite shape �No definite shape �Definite volume �Particles move fast enough to separate a little Allowing them to change shape �Particles do not move fast enough to change volume

Liquid (Continued) �Viscosity: a liquid’s resistance to flow High Viscosity: Slow Flow (Honey) Low Viscosity: Fast Flow (Water) �Surface Tension: force that attracts the molecules at the surface of a liquid to form the drop

Gas �The state of matter that does not have a definite shape or volume �No definite shape �No definite volume �Particles move so fast that they completely separate from each other Allowing them to change shape and volume
Changes of State �Change of a substance from one physical form to another �Requires adding or removing energy so that particles can speed up or slow down
Melting �Change of state when a solid becomes a liquid �Particles must speed up �Add energy/heat �Endothermic �Melting Point: Temperature at which a substance melts Water: 32°F or 0°C

Freezing �Change of state from a liquid to a solid �Particles must slow down �Remove energy/heat �Exothermic �Freezing Point: Temperature at which a substance freezes Water: 32°F or 0°C

Boiling �Change of a liquid to a gas (throughout an entire liquid) �Particles (on bottom of liquid) must speed up �Add energy/heat �Endothermic �Boiling Point: Temperature at which a substance boils Water: 212°F or 100°C At Sea Level: Boiling depends on Air Pressure **Won’t happen unless air pressure equals pressure in bubbles

Evaporation �Change of a substance from a liquid to a gas (only on surface) �Particles (on surface) must speed up �Add energy/heat �Endothermic
Condensation �Change of state from a gas to a liquid �Particles must slow down �Remove energy/heat �Exothermic

Sublimation �Change of state from a solid to a gas Example: Dry Ice �Skips liquid stage �Add energy/heat �Endothermic
- Slides: 15