CHEMISTRY UNIT CHAPTER 3 Periodic Table Part 1

CHEMISTRY UNIT: CHAPTER 3 Periodic Table Part 1

Dmitri Mendeleev • Noticed some elements behaved the same • Wondered if they could be organized by any specific pattern • Focused on melting point, density, color, atomic mass • Published first periodic table of 63 elements in 1869
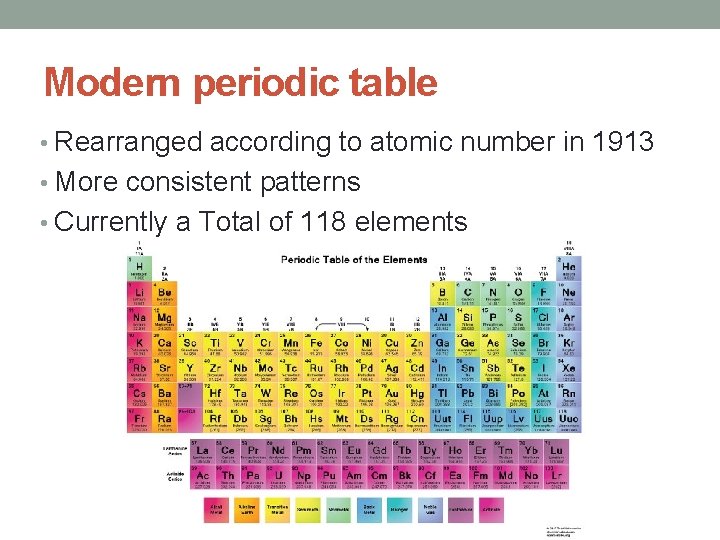
Modern periodic table • Rearranged according to atomic number in 1913 • More consistent patterns • Currently a Total of 118 elements

How do we find an element? • Periods • Horizontal rows going left and right • Period numbers are down the left side • Groups • Vertical columns going down • Group numbers are across the top of the periodic table • Use them like battleship
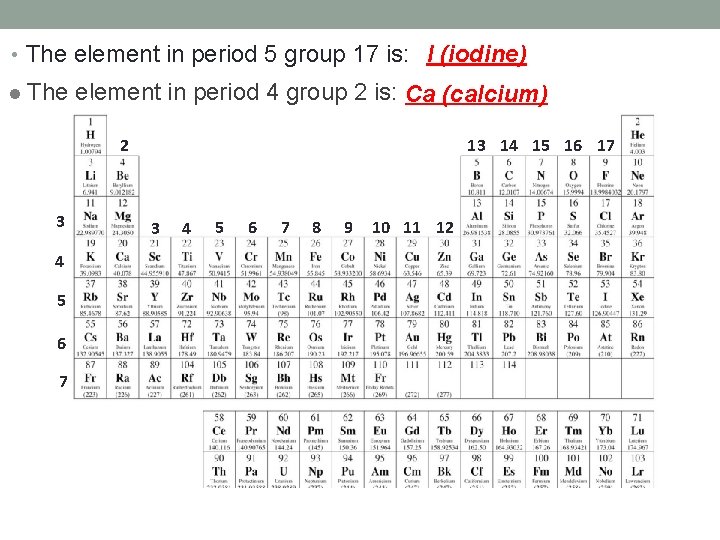
• The element in period 5 group 17 is: I (iodine) l The 1 element in period 4 group 2 is: Ca (calcium) 1 2 13 14 15 16 17 2 3 4 5 6 7 8 9 10 11 12 18

How do we read an element’s square? • Always the same information • Read from top to bottom • Tells you 7 things
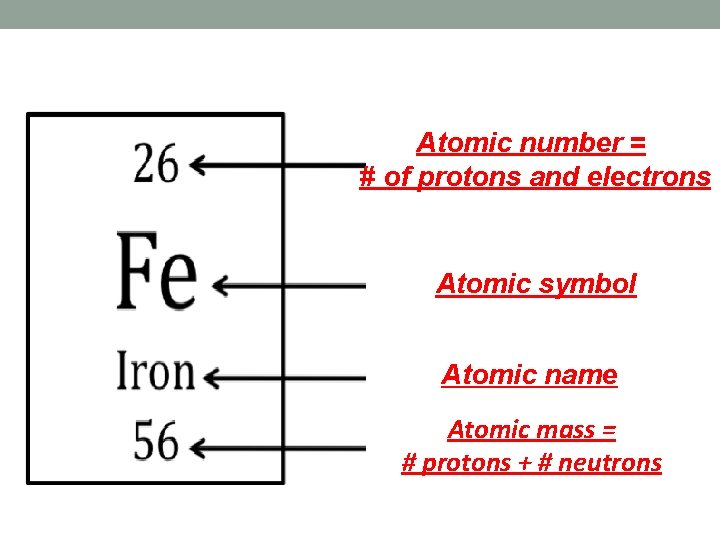
Atomic number = # of protons and electrons Atomic symbol Atomic name Atomic mass = # protons + # neutrons
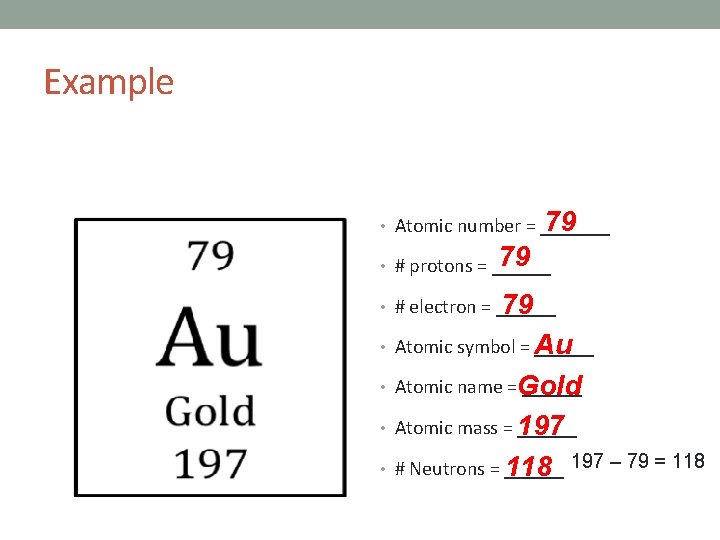
Example 79 • Atomic number = _______ 79 • # protons = ______ 79 • # electron = ______ Au • Atomic name =Gold ______ • Atomic mass = 197 ______ • # Neutrons = ______ 118 197 – 79 = 118 • Atomic symbol = ______
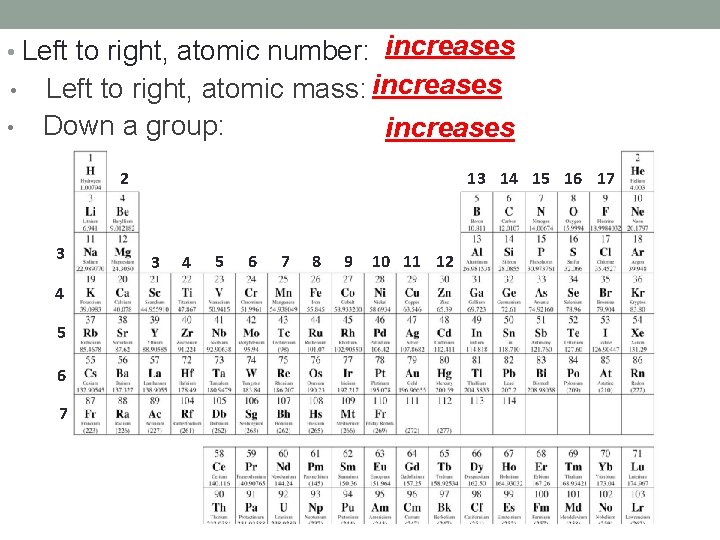
• Left to right, atomic number: increases • • Left to right, atomic mass: increases Down a group: increases 1 1 2 13 14 15 16 17 2 3 4 5 6 7 8 9 10 11 12 18
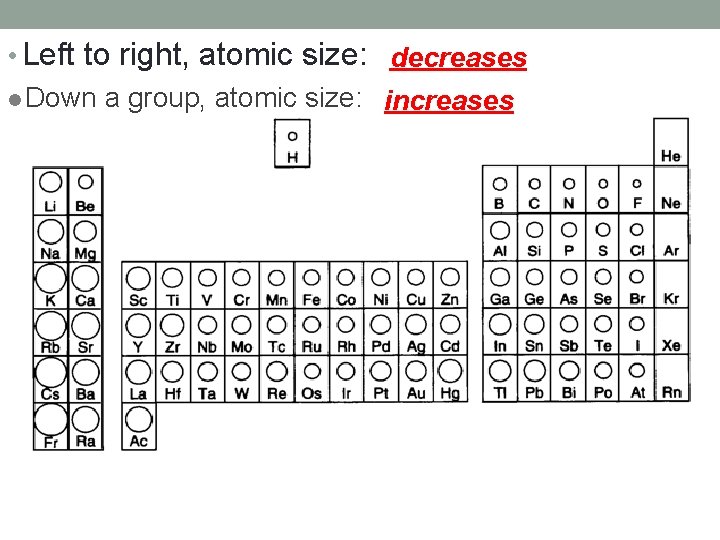
• Left to right, atomic size: decreases l Down a group, atomic size: increases

- Slides: 11