Chemistry Unit 8 Thermochemistry Chapter 17 17 1


















- Slides: 31

Chemistry Unit 8 Thermochemistry Chapter 17

17. 1 The Flow of Energy Transformations – Goal 1 • Chemical Potential Energy • Energy stored in chemical bonds • Measured in Joules (j) • Determined by: – Kinds of Atoms – Arrangement of Atoms • Heat (q) • Measure of the total amount of energy (E) transferred from an object with high E to an object with low E • Also measured in Joules (j) • Temperature (T) • • Measure of average kinetic E of the particles Indicates relative amounts of E Indicates the hotness or coldness of an object Measured in Kelvin, Celsius, or Fahrenheit

Which has more energy? • Cup of water at 30°C or tub full of water at 30°C? • Cup of water at 100°C or tub full of water at 30°C? • What type of energy is stored in the water? – Chemical Potential Energy • Locked up (only released by reactions) – Kinetic Energy • Easily experienced in our everyday lives

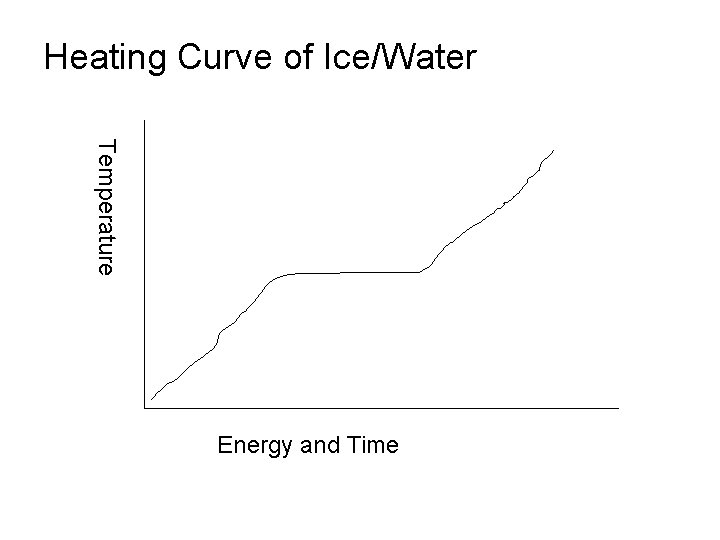
• Energy Concepts: • System: Substance as Solid, liquid, or gas • Surroundings: Environment around Substance • Definitions of physical State Changes: • Melting • Boiling • Condensing • Freezing • Subliming

Heating Curve of Ice/Water Temperature Energy and Time

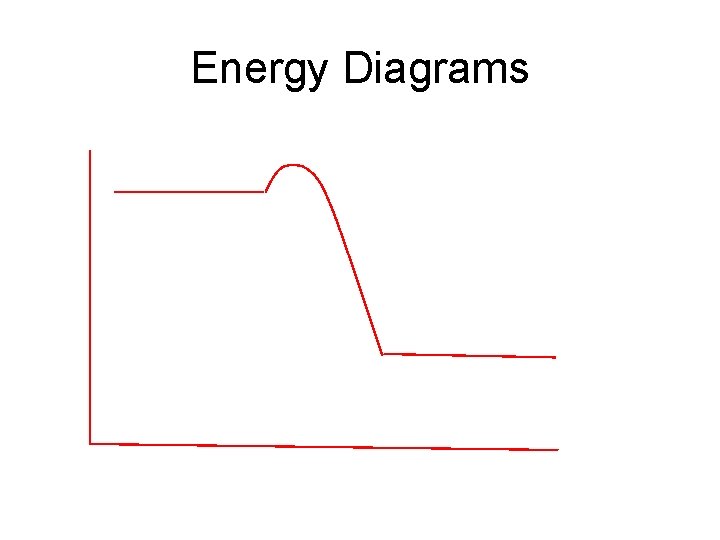
Endothermic and Exothermic Processes – Goal 2 • Law of Conservation of Energy • In a chemical reaction, energy is neither created nor destroyed. • Enthalpy (H) • Total Energy of a system • (Mostly Potential Energy & Kinetic Energy) • Enthalpy Change (ΔH) • Energy change of a process (physical or chemical) ΔH = H (Products) – H (Reactants) • If at constant pressure: Heat released or absorbed is Enthalpy Change ΔH = q

Types of Enthalpy Changes • Losing energy: Exothermic • • • Products have less energy than the reactants ΔH = small – large = (-) value Δ H = (-) System LOSES energy Surroundings GAIN energy • Gaining energy: Endothermic • • • products have more energy then reactants Δ H = large – small = (+) value Δ H = (+) System GAINS energy Surroundings LOSE energy

Energy Diagrams

Examples • State Changes • • Melting? Freezing? Condensing? Evaporation or Boiling? • Chemical Reactions • Combustion? • Heat Pack? • Cold Pack?

Heat Capacity & Specific Heat – Goal 3 • Heat Capacity • Amount of energy needed to raise the temperature of an object • Example: » A large body of water (Ocean) has a greater Heat Capacity than a small pond • Specific Heat Capacity (c) • Amount of energy needed to raise one gram of a substance 1 °C • Units: joules per (gram °C) or J/g°C • Table p. 296 • “Specific” to a substance: Each substance has it’s own unique Specific Heat Capacity.

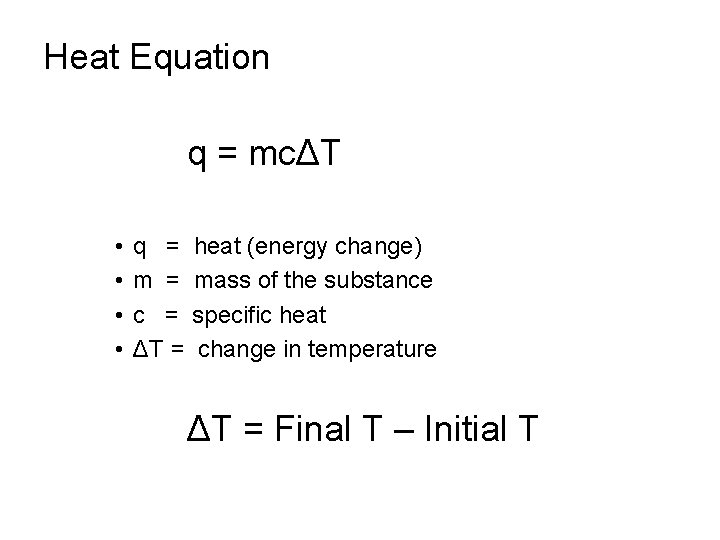
Heat Equation q = mcΔT • • q = m = c = ΔT = heat (energy change) mass of the substance specific heat change in temperature ΔT = Final T – Initial T

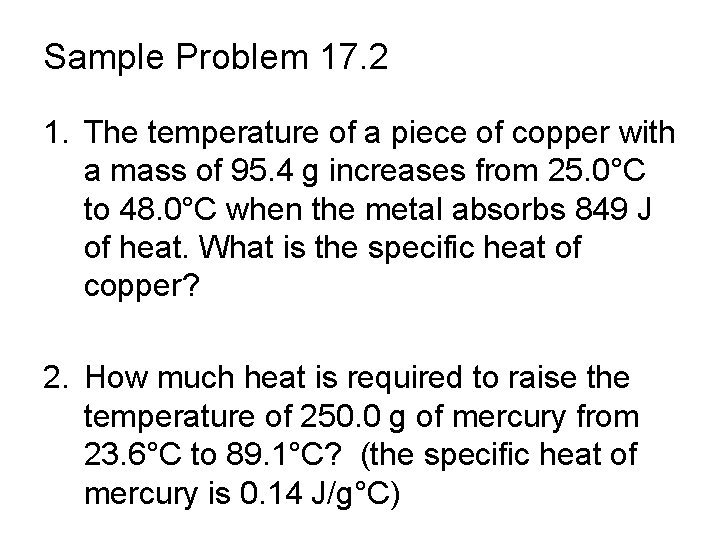
Sample Problem 17. 2 1. The temperature of a piece of copper with a mass of 95. 4 g increases from 25. 0°C to 48. 0°C when the metal absorbs 849 J of heat. What is the specific heat of copper? 2. How much heat is required to raise the temperature of 250. 0 g of mercury from 23. 6°C to 89. 1°C? (the specific heat of mercury is 0. 14 J/g°C)

17. 2 Measuring and Expressing Enthalpy Changes Calorimetry – Goal 4 • Calorimeter • An instrument used to measure the heat changes of a reaction or process • Qualities: – Well Insulated – Uses a known substance (i. e. water) – Use the specific heat (c) and ∆T to solve for Heat • Example: Lab • Heat water with a process • Measure: 1) mass of water 2) ∆T of water 3) Know c • Calculate: 1) Heat absorbed by water • Conservation of Energy Heat absorbed by water = Heat lost by Process

Sample Problem 17. 3 • When 25. 0 m. L of water containing 0. 025 mol HCl at 25˚C is added to 25. 0 m. L of water containing 0. 025 mol Na. OH at 25. 0˚C in a foam cup calorimeter, a reaction occurs. Calculate the enthalpy change (in k. J) during this reaction if the highest temperature observed is 32. 0˚C. Assume the densities of the solutions are 1. 00 g/m. L and the volume of the final solution is equal to the sum of the volumes of reacting solutions.

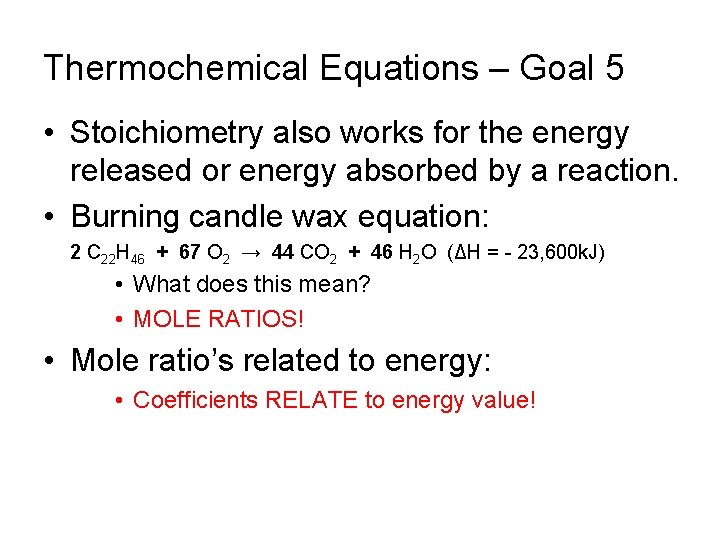
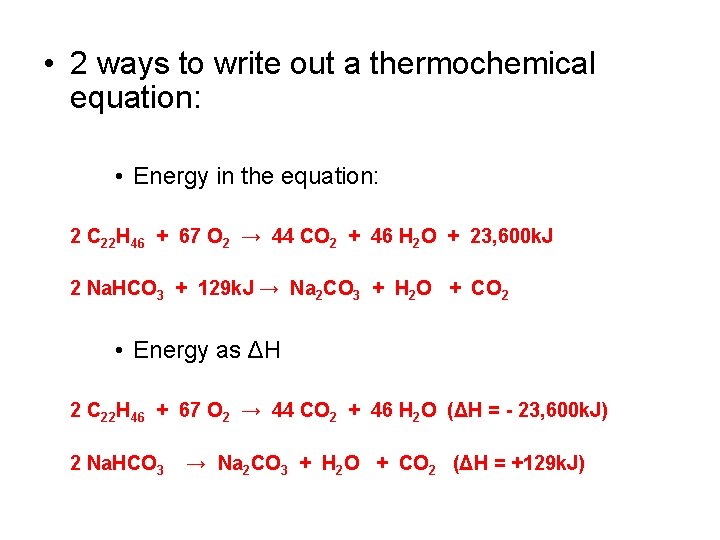
Thermochemical Equations – Goal 5 • Stoichiometry also works for the energy released or energy absorbed by a reaction. • Burning candle wax equation: 2 C 22 H 46 + 67 O 2 → 44 CO 2 + 46 H 2 O (ΔH = - 23, 600 k. J) • What does this mean? • MOLE RATIOS! • Mole ratio’s related to energy: • Coefficients RELATE to energy value!

• 2 ways to write out a thermochemical equation: • Energy in the equation: 2 C 22 H 46 + 67 O 2 → 44 CO 2 + 46 H 2 O + 23, 600 k. J 2 Na. HCO 3 + 129 k. J → Na 2 CO 3 + H 2 O + CO 2 • Energy as ΔH 2 C 22 H 46 + 67 O 2 → 44 CO 2 + 46 H 2 O (ΔH = - 23, 600 k. J) 2 Na. HCO 3 → Na 2 CO 3 + H 2 O + CO 2 (ΔH = +129 k. J)

2 C 22 H 46 + 67 O 2 → 44 CO 2 + 46 H 2 O (ΔH = - 23, 600 k. J) Example Problems: 1) How much energy is released when 50. 0 g of candle wax burns? 2) How much energy is released when 1 mol of candle wax burns? 3) How much energy is released when 1 g of candle wax burns?

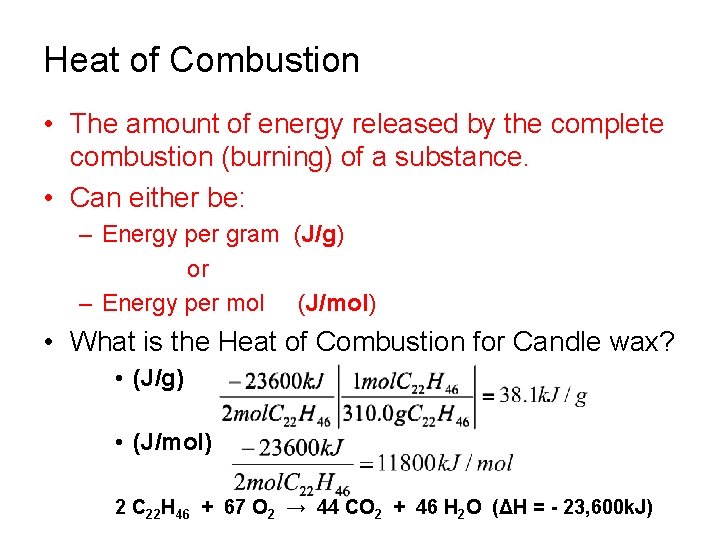
Heat of Combustion • The amount of energy released by the complete combustion (burning) of a substance. • Can either be: – Energy per gram (J/g) or – Energy per mol (J/mol) • What is the Heat of Combustion for Candle wax? • (J/g) • (J/mol) 2 C 22 H 46 + 67 O 2 → 44 CO 2 + 46 H 2 O (ΔH = - 23, 600 k. J)

17. 3 Heat in Changes of State – Goal 6 • Heat can change 2 things for a substance • Change Temperature ( q = mcΔT ) • Change State (new) • State Changes require Energy • Change from Solid to Liquid » Heat of Fusion » Opposite? » Heat of solidification (opposite value of fusion) • Change from Liquid to Gas » Heat of Vaporization » Opposite? » Heat of Condensation (opposite value of Vapor)

Heat of Fusion • Symbol: ΔHfus • Energy required to change 1 g of solid to liquid • Equation • ΔHfus = q/m or q = (m)(ΔHfus) • Water: ΔHfus = 333 J/g ΔHfus = +333 J/g (melting) ΔHsol = -333 J/g (Solidification)

Heat of Vaporization • Symbol: ΔHvap • Energy required to change 1 g of Liquid to gas • Equation • ΔHvap = q/m or q = (m)(ΔHvap) • Water: ΔHvap = 2260 J/g ΔHvap = +2260 J/g (Vaporizing) ΔHcon = -2260 J/g (Condensation)

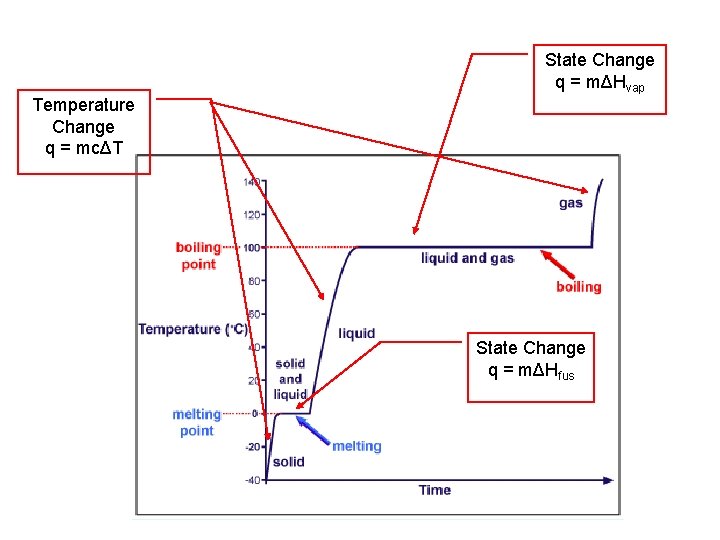
State Change q = mΔHvap Temperature Change q = mcΔT State Change q = mΔHfus

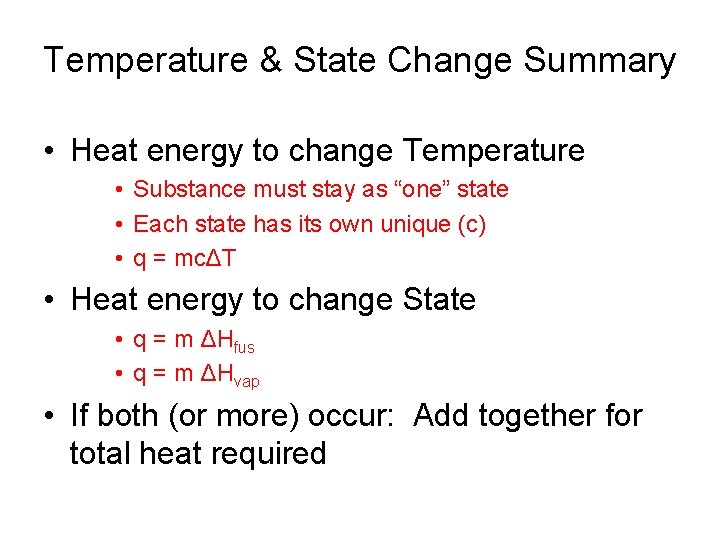
Temperature & State Change Summary • Heat energy to change Temperature • Substance must stay as “one” state • Each state has its own unique (c) • q = mcΔT • Heat energy to change State • q = m ΔHfus • q = m ΔHvap • If both (or more) occur: Add together for total heat required

Example Problems 1. How much heat energy is required to completely freeze 50. 0 g of water that is at (+20˚C)? 2. How much heat energy is required to change 125 g of ice at -12°C to a gas at 108°C?

Algebra Review A + 2 B = 18 A–B=3

17. 4 Hess’s Law – Goal 7 • Thermochemical Review • Heat of Reaction • Heat released or absorbed for a known chemical reaction • Heat (ΔH) depends on the Balanced Equation • (ΔH)=(-): Heat was released • (ΔH)=(+): Heat was absorbed • Hess’s Law explains how to _____the Heat of Reaction from other known reactions

17. 4 Hess’s Law – Goal 7 • Thermochemical Review • Heat of Reaction • Heat released or absorbed for a known chemical reaction • Heat (ΔH) depends on the Balanced Equation • (ΔH)=(-): Heat was released • (ΔH)=(+): Heat was absorbed • Hess’s Law explains how to “calculate” the Heat of Reaction from other known reactions

Hess’s Law: Concepts • Equations can be added together to obtain a new equation. • The heats of reaction (ΔH) must also be added to obtain the new equations heat of reaction (ΔH). • Known equations may be reversed or multiplied by a factor so that they will “add” together for the needed result.

Hess’s Law: Rules • If an Equation is reversed, then its ΔH is opposite (change the sign). • Some equations may need to be multiplied by a factor, if so then their ΔH also need to be multiplied by the factor.

Hess’s Law: Example • What is the Heat of Reaction for: Cdiamond Cgraphite • You are given: Cgraphite + O 2 CO 2 Cdiamond + O 2 CO 2 ΔH = -393. 5 k. J ΔH = -395. 4 k. J CO 2 Cgraphite + O 2 Cdiamond + O 2 CO 2 ΔH = +393. 5 k. J ΔH = -395. 4 k. J Cdiamond Cgraphite ΔH = • Reverse • Add

Hess’s Law: Example If: PCl 5 PCl 3 + Cl 2 2 P + 3 Cl 2 2 PCl 3 What is the ∆H of: 2 P + 5 Cl 2 2 PCl 5 ∆H= 87. 9 k. J ∆H= -574 k. J