CHEMISTRY the study of matter MATTER anything that


















- Slides: 20

CHEMISTRY the study of matter

MATTER anything that has mass and has volume

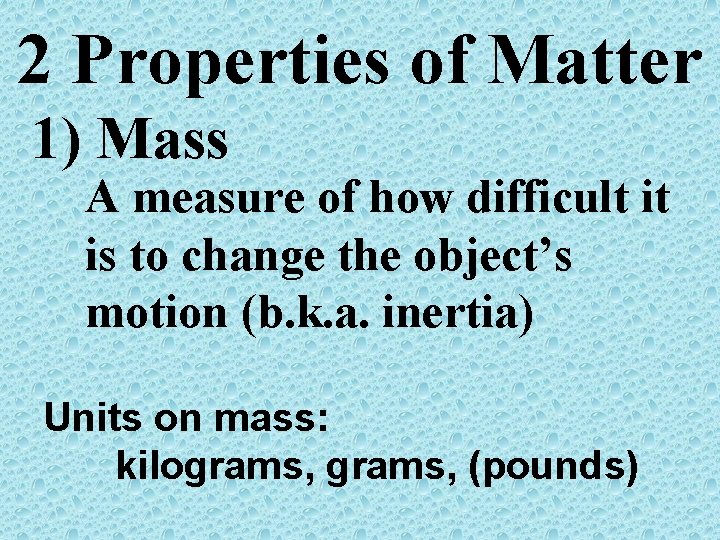
2 Properties of Matter 1) Mass A measure of how difficult it is to change the object’s motion (b. k. a. inertia) Units on mass: kilograms, (pounds)

Massive objects are harder to move. Thus they have more matter and more inertia Which has more inertia?

Weight vs. Mass • Weight Depends on gravity (changes depending on where you are) • Mass Stays the same EVERYWHERE!!!

2 Properties of Matter 2) Volume The amount of space an object occupies Units on volume: milliliters, (cup, gallon)

An object’s volume can be found in 4 basic ways: 1. Multiplying dimensions 2 3 together (lwh, πr h, 4/3 πr ) • Volume displacement • Using a liquid measure (m. L) • As a derived unit (D=m/v)

Density The ratio of an object’s mass to its volume

Units: g/m. L, 3 g/cm

Anything with a density of GREATER than 1 g/m. L will sink in water. Anything with a density LESS than 1 g/m. L will float in water.

A block of wood has a mass of 28 grams. If 3 its volume is 14 cm , what is the density of the wood? 2. 0 3 g/cm

The density of gold is 19. 3 g/m. L. What is the mass of a piece of gold with a volume of 34. 5 m. L? 666 grams

Air has a density of 0. 0013 g/m. L. How much volume does 48, 000 grams of air take up? 3. 7 x 7 10 m. L

PROBLEM What is the density of sand?

PROBLEM Are your 10 pennies made before 1982 or after 1982?

Accuracy How close a measurement is the correct or true measurement

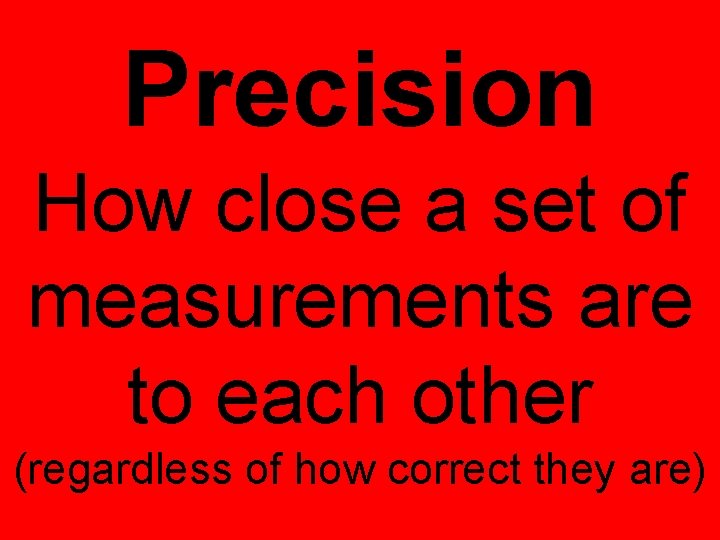
Precision How close a set of measurements are to each other (regardless of how correct they are)

% Error A quantitative comparison between your value and an accepted value

Your value for water density = 0. 88 Accepted value for water density = 1. 0 Calculate the % error in your measurement!

 Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Block xoang nhĩ là gì
Block xoang nhĩ là gì Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Mass
Mass Matter is anything that has
Matter is anything that has Anything that has mass and takes up space
Anything that has mass and takes up space Matter anything that
Matter anything that Matter is defined as anything that
Matter is defined as anything that What is anything that has mass and volume
What is anything that has mass and volume Anything that takes up space
Anything that takes up space Anything that takes up space and has mass
Anything that takes up space and has mass Anything that occupies space
Anything that occupies space What is anything that has mass and takes up space
What is anything that has mass and takes up space Matter anything that
Matter anything that