Chemistry The Study of Matter Chemistry is the





















































































































- Slides: 149

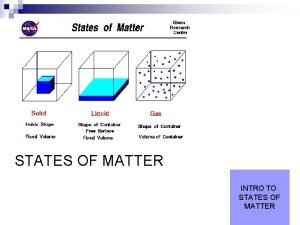
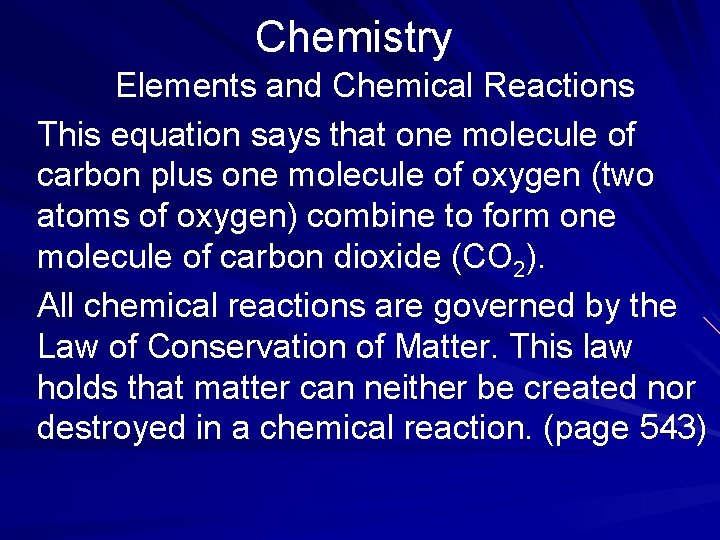
Chemistry The Study of Matter Chemistry is the branch of science that deals with the composition, structure, and properties of matter as well as the changes it undergoes. Matter is any substance that occupies space and has mass. Your chair, desk, and table are composed of matter. (page 533)

Chemistry The Study of Matter Even the air you breathe is composed of matter. Matter exists in four states— solid, liquid, gas, and plasma (an ionized gas of which the Sun is made). The starting point for a systematic study of chemistry generally begins with an examination of the basic unit of matter, the atom. (page 533)

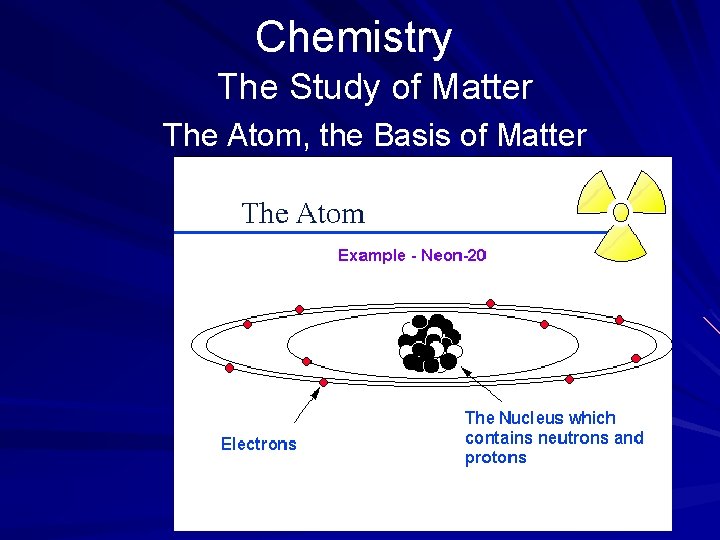
Chemistry The Study of Matter The Atom, the Basis of Matter In chemistry, atoms are the building blocks for matter. The atom is the smallest particle of an element that has the properties of that element. An element is a substance that occurs in nature and that cannot be broken down into a simpler substance. (page 533)

Chemistry The Study of Matter The Atom, the Basis of Matter Nearly 100 fundamental substances known as elements are known to occur in nature. A few elements have been produced synthetically by man. Atoms also form molecules. A molecule is the smallest part of a compound that can exist by itself. A molecule consists of two or more atoms joined together chemically. (page 533)

Chemistry The Study of Matter The Atom, the Basis of Matter

Chemistry The Study of Matter The Atom, the Basis of Matter

Chemistry The Study of Matter The Atom, the Basis of Matter

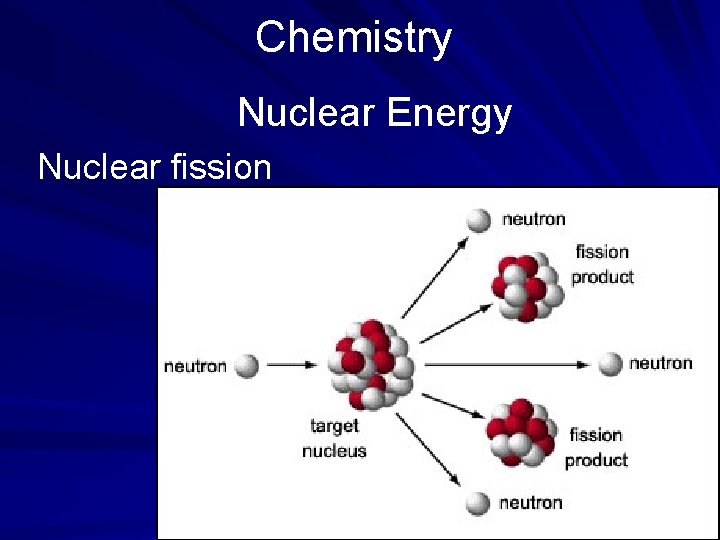
Chemistry The Study of Matter The Atom, the Basis of Matter In the early 19 th century, only a few elements were known to exist. According to theory of John Dalton, an atom cannot be made, destroyed, or divided; and atoms of the same element are alike. This concept became known as atomic theory. Later physicists discovered that the nucleus of an atom can be split by bombarding it with neutrons, a process known as nuclear fission. (page 534)

Chemistry The Study of Matter The Atom, the Basis of Matter John Dalton

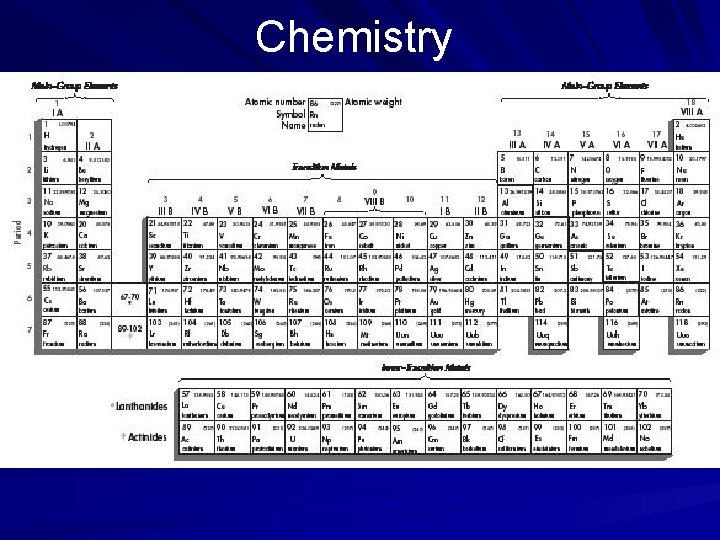
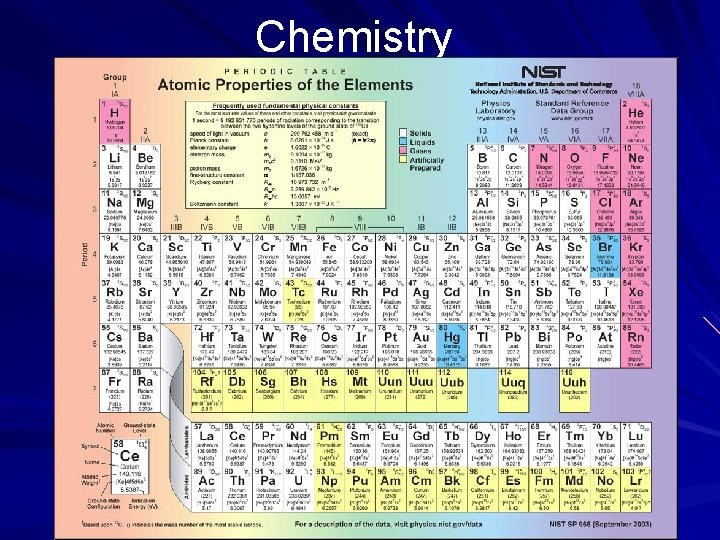
Chemistry The Study of Matter The Atom, the Basis of Matter A Russian chemist, Dmitri Mendeleyev, constructed a table, known as the periodic table, by which he calculated the atomic weights of the different elements. The elements are identified by symbols taken largely from Latin names for the elements. Hydrogen is the lightest known element, having only one proton and was, therefore, assigned the atomic number 1. (page 534)

Chemistry The Study of Matter The Atom, the Basis of Matter Dmitri Mendeleyev

Chemistry

Chemistry

Chemistry The Study of Matter The Atom, the Basis of Matter An atom of oxygen, an abundant gas on Earth, has a mass 16 times that of a hydrogen atom; therefore, oxygen was given an atomic mass of 16. Oxygen is the eighth lightest element and is assigned the atomic number 8. (page 534)

Chemistry The Study of Matter The Atom, the Basis of Matter Dalton's and Mendeleyev's discoveries were the most significant in the field of chemistry since that of Antoine-Laurent Lavoisier, a French chemist who identified oxygen as the key element that supports combustion. (page 534)

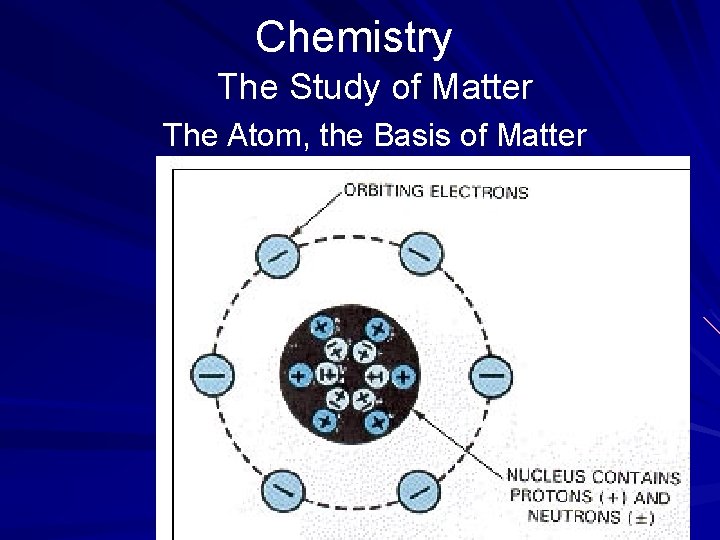
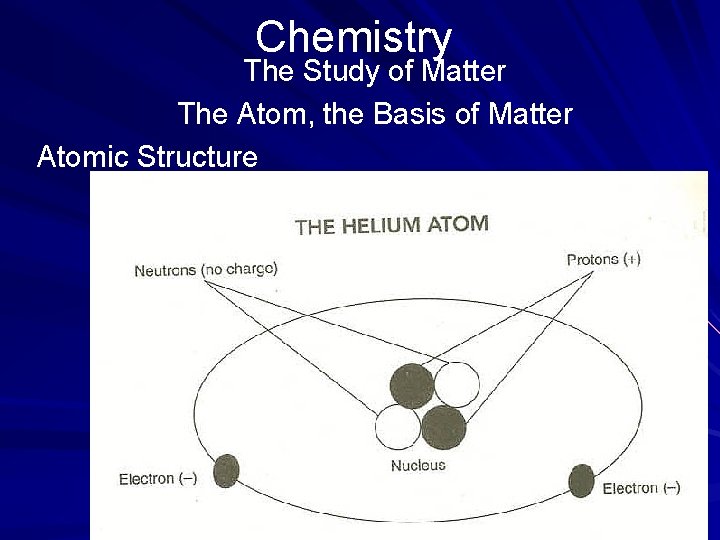
Chemistry The Study of Matter The Atom, the Basis of Matter Atomic Structure Scientists have learned a great deal about atoms since Dalton's time. For example, an atom is composed of a nucleus with electrons that surround it. The nucleus, located in the center of the atom, is made up of protons and neutrons. (page 534)

Chemistry The Study of Matter The Atom, the Basis of Matter Atomic Structure A proton is a positively charged particle. An element's atomic number is determined by the number of protons it has. Because hydrogen has only one proton in its nucleus, it has an atomic number of 1. A neutron has a mass nearly equal to that of a proton but has no charge at all. The nucleus has a positive charge, determined by the number of protons it contains. The nucleus provides the mass number for an element. (page 534)

Chemistry The Study of Matter The Atom, the Basis of Matter Atomic Structure An electron is a negatively charged particle. Electrons occupy an orbit, or shell, that surrounds the nucleus. Each shell can hold only a fixed number of electrons. It is the number of shells that distinguishes one element from another. The greater the number of shells with orbiting electrons that an element has, the greater its atomic number. (page 534)

Chemistry The Study of Matter The Atom, the Basis of Matter Atomic Structure

Chemistry EXERCISE 1 Atomic Structure Directions: Match each term on the right with the correct description on the left. (page 535) 1. ____ 2. ____ 3. ____ 4. ____ 5. ____ 6. ____ the second lightest element; contains two protons in its nucleus a negatively charged particle the part of an atom that determines an element's mass a particle that has no charge a positively charged particle an element containing eight protons in its nucleus a. oxygen b. neutron c. proton d. helium e. electron f. nucleus

Chemistry Nuclear Energy The nucleus of every atom contains an almost unimaginable amount of potential energy. The protons that are locked together are all positive and naturally repel each other. It takes the strongest force in the universe, nuclear force, to keep those subatomic particles locked together. (page 536)

Chemistry Nuclear Energy Nuclear fission

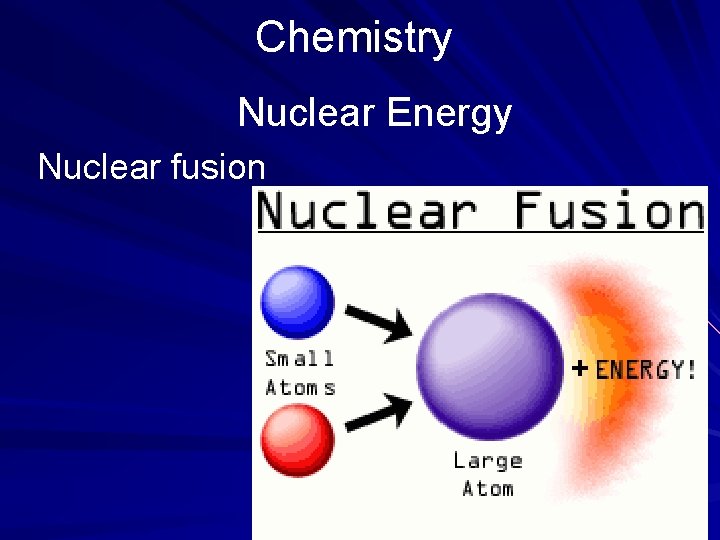
Chemistry Nuclear Energy Nuclear fusion

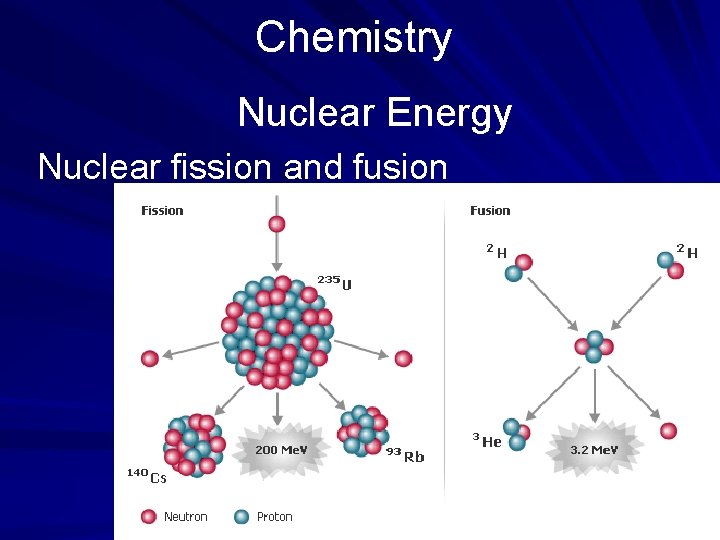
Chemistry Nuclear Energy Nuclear fission and fusion

Chemistry Nuclear Energy Science has been able to unlock and capture this energy by splitting larger atoms (those with the greatest number of protons) by firing a neutral neutron at the atom. This process of splitting the larger atoms is nuclear fission. The fuel for this reaction is uranium because of the great size of its nucleus and its unstable qualities. (page 536)

Chemistry Nuclear Energy When a material is unstable and able to release radiation it is said to be radioactive. This radioactive material is made into pellets that are held by fuel rods placed in a heavily shielded nuclear reactor. (page 536)

Chemistry Nuclear Energy

Chemistry Nuclear Energy The process of regulating the release of the nuclear energy requires that the fuel rods remain partially covered with control rods. These control rods prevent free neutrons from splitting too many uranium atoms in an uncontrolled explosion. (page 536)

Chemistry Nuclear Energy Radioactivity and Environmental Protection One of the dangers of nuclear energy is that such an explosion could release radioactive materials into the environment, causing serious and extensive contamination of radioactivity to any living organisms, plants and animals in the vicinity. (page 536)

Chemistry Nuclear Energy Radioactivity and Environmental Protection Chernobyl

Chemistry Nuclear Energy Radioactivity and Environmental Protection Chernobyl

Chemistry Nuclear Energy Radioactivity and Environmental Protection Chernobyl

Chemistry Nuclear Energy Radioactivity and Environmental Protection Chernobyl

Chemistry Nuclear Energy Radioactivity and Environmental Protection One such explosion occurred at a Ukranian nuclear power plant, Chernobyl, in the early 1980 s. A combination of poor operator judgment and a tight testing schedule created the conditions that caused too many of the fuel rods to be exposed. The overwhelming heat from the reaction melted through the containment walls, and an explosion sent radioactive particles and gases outside to the nearby forest and town. (page 536)

Chemistry Nuclear Energy Radioactivity and Environmental Protection To stop the fire and prevent further leakage, the entire reactor was encased in cement, which is now referred to as the sarcophagus (a stone coffin). Local animals that were contaminated as well as the entire nearby forest and even construction equipment used during the cleanup had to be buried as well. (page 536)

Chemistry Nuclear Energy Radioactivity and Environmental Protection Evacuation of the residents of the town near the reactor did not allow the packing of personal belongings. The town remains empty to this day, and the objects left behind convey a very eerie "ghost town" effect. (page 536)

Chemistry Nuclear Energy Radioactivity and Environmental Protection The United States also has experienced the fear of a near disaster. A nuclear power plant at Three Mile Island, located in New Jersey, sprang a leak of cooling water. This leak allowed the reactor to become very hot. (page 536)

Chemistry Nuclear Energy Radioactivity and Environmental Protection Three Mile Island

Chemistry Nuclear Energy Radioactivity and Environmental Protection The American reactor, however, was equipped with an automatic shut-off system that was activated when the recorded temperature exceeded maximum capacity. There was no leakage of radioactive materials, and nuclearenergy advocates explain that this fact proves that the automatic system keeps nuclear power safe. (page 536)

Chemistry Nuclear Energy Radioactivity and Environmental Protection These advocates still do not have an answer to the biggest concern nuclear-energy opponents have: the safe disposal of radioactive wastes. Currently these wastes are sealed in large barrels and are transported to empty underground mines that are reinforced with a lining to protect the environment. (page 536)

Chemistry EXERCISE 2 Nuclear Energy Directions: Read the passage below and choose the best answer for each of the following items. You may need to refer to the periodic table shown on page 541. Nuclear energy may be released in two ways: by fission and by fusion. Nuclear fission involves the splitting of the nucleus of a heavy chemical element by bombardment with neutrons. Nuclear fusion involves the uniting of two nuclei of an element at high temperatures and pressure to form the nucleus of a new, heavier, element. In each process, nuclear energy is released. (page 537)

Chemistry EXERCISE 2 Nuclear Energy (page 537) 1. According to the information in the passage and the atomic masses shown in the periodic table, when would energy from nuclear fusion be released? (1) when uranium nuclei are fused to make plutonium (2) when hydrogen nuclei are fused to make oxygen (3) when oxygen nuclei are fused to make helium (4) when hydrogen nuclei are fused to make helium (5) when helium nuclei are fused to make hydrogen

Chemistry EXERCISE 2 Nuclear Energy (page 537) 2. Energy from nuclear fission would be released in the splitting of the nucleus of which element? (1) plutonium (2) hydrogen (3) oxygen (4) helium (5) carbon

Chemistry EXERCISE 2 Nuclear Energy (page 537) 3. Identify the following statements about nuclear power as true (T) or false (F). _____ The Three Mile Island accident contaminated a nearby forest. _____ Nuclear waste is safe to dispose of in landfills. _____ The Chernobyl accident could have been prevented. _____ Fuel rods must also have control rods to control the reaction.

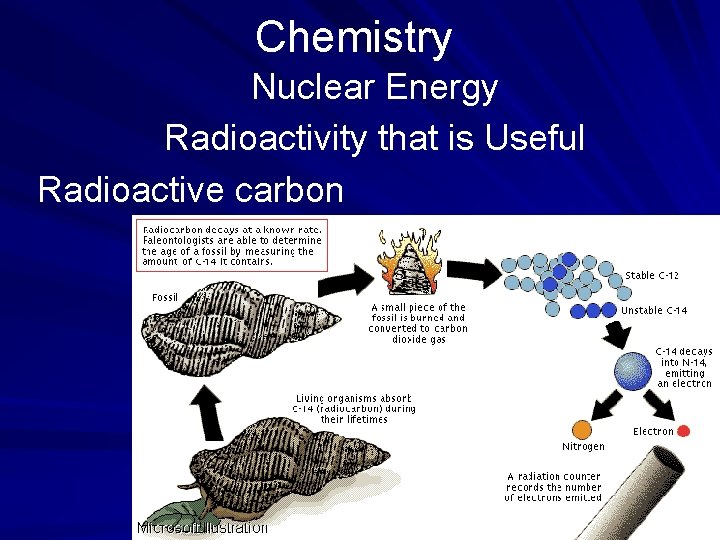
Chemistry Nuclear Energy Radioactivity that is Useful Not all radioactivity is harmful. Particles of radioactive carbon are in the air and inhaled by animals and people every day. It is this radioactive carbon that allows paleontologists to calculate how long an organism has been dead. (page 538)

Chemistry Nuclear Energy Radioactivity that is Useful Radioactive carbon

Chemistry Nuclear Energy Radioactivity that is Useful Once the organism dies, it no longer takes in additional radioactive C 14, and the amount of radioactive carbon that is present in the organism starts to decay or lose some of its radioactivity in the form of subatomic particles called alpha or beta particles. (page 538)

Chemistry Nuclear Energy Radioactivity that is Useful The release of alpha and beta particles would contaminate nearby objects with radioactivity. This process of radiocarbon dating has given scientists a tool to examine and date fossils from once-living organisms including early man. (page 538)

Chemistry Nuclear Energy Radioactivity that is Useful Smoke detectors also have a small, safe amount of material that gives off alpha particles. Some smoke detectors contain small amounts of Am 241 (Americium) that releases a steady stream of alpha particles between two electrodes. When smoke particles interrupt the current between the two electrodes, an alarm sounds. (page 538)

Chemistry Nuclear Energy Radioactivity that is Useful Radioactive materials are also used in the treatment of some cancers. An entire branch of medicine, called nuclear medicine, researches and uses radioactive materials to treat the human body. (page 538)

Chemistry Nuclear Energy Radioactivity that is Useful Marie Curie

Chemistry Nuclear Energy Radioactivity that is Useful One of the first medical explorers to use radioactivity was Marie Curie. In 1903 she and her husband, Pierre, won the Nobel Prize for their research. Unfortunately, they did not understand the harmful effects of radiation until they had both suffered permanent physical damage from their long exposure to it. (page 538)

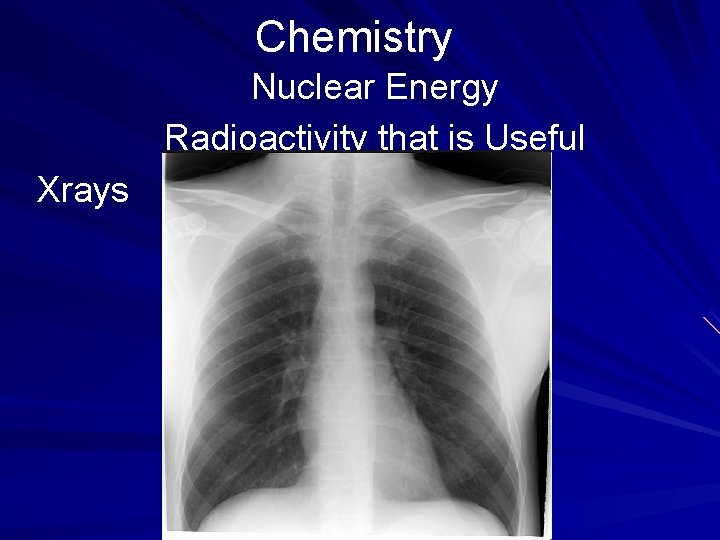
Chemistry Nuclear Energy Radioactivity that is Useful One of the most popular forms of radiology involves the use of Xrays to detect broken bones. While having Xrays at the doctor's or dentist's office, the patient is also given a protective lead cover to prevent overexposure of the other parts of the body. (page 538)

Chemistry Nuclear Energy Radioactivity that is Useful Xrays

Chemistry Nuclear Energy Radioactivity that is Useful Xrays

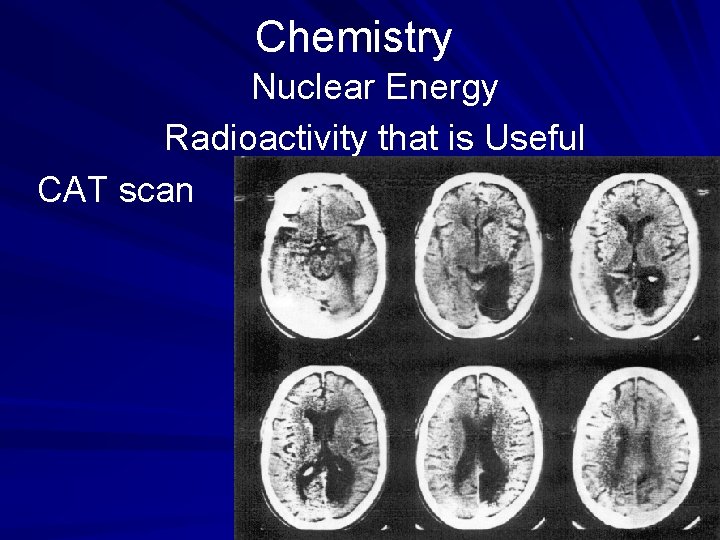
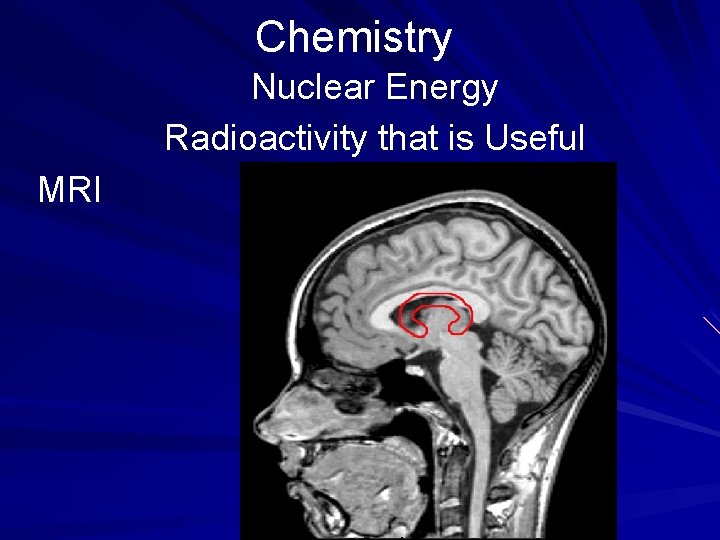
Chemistry Nuclear Energy Radioactivity that is Useful CAT (computerized axial tomography) scans use computers to monitor the body's reaction to Xrays from a variety of angles. Doctors use CAT scans as well as MRI (magnetic resonance imaging) screens to help in diagnosis of internal problems. (page 538)

Chemistry Nuclear Energy Radioactivity that is Useful CAT scan

Chemistry Nuclear Energy Radioactivity that is Useful MRI

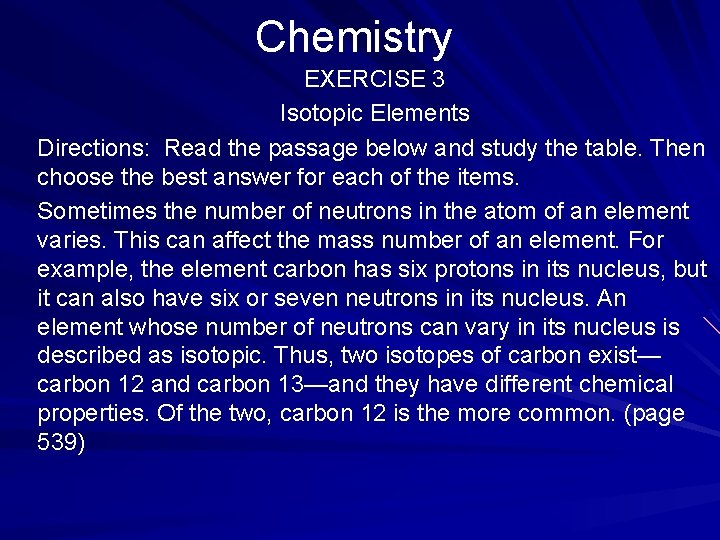
Chemistry EXERCISE 3 Isotopic Elements Directions: Read the passage below and study the table. Then choose the best answer for each of the items. Sometimes the number of neutrons in the atom of an element varies. This can affect the mass number of an element. For example, the element carbon has six protons in its nucleus, but it can also have six or seven neutrons in its nucleus. An element whose number of neutrons can vary in its nucleus is described as isotopic. Thus, two isotopes of carbon exist— carbon 12 and carbon 13—and they have different chemical properties. Of the two, carbon 12 is the more common. (page 539)

Chemistry EXERCISE 3 Isotopic Elements Element Atomic Number Mass Number Hydrogen 1 1. 01 Helium 2 4 Lithium 3 6. 94 Beryllium 4 9. 01 Boron 5 10. 81

Chemistry EXERCISE 3 Isotopic Elements (page 539) 1. The only element in the chart that could have an isotope of mass number 6 and whose two nuclei might be fused to form carbon 12 would be which of the following? (1) hydrogen (2) helium (3) lithium (4) beryllium (5) boron

Chemistry EXERCISE 3 Isotopic Elements (page 539) 2. Deuterium and tritium are two isotopes that have mass numbers of 2 and 3, respectively. Of the two isotopes, tritium is especially radioactive. Based on the preceding chart, to which element would these two isotopes belong, knowing that they both have only one proton? (1) hydrogen (2) helium (3) lithium (4) beryllium (5) boron

Chemistry EXERCISE 3 Isotopic Elements (page 539) 3. Identify the following statements as either fact (F) or opinion (O). ___ Radioactivity is very dangerous, and people should not use it. ___ Radiation can be used to treat certain medical problems. ___ Lead is used as a screen to protect internal organs when using Xrays. ___ CAT scans and MRIs are the best ways to diagnose disease. ___Radioactive wastes need to be securely protected from contaminating the environment.

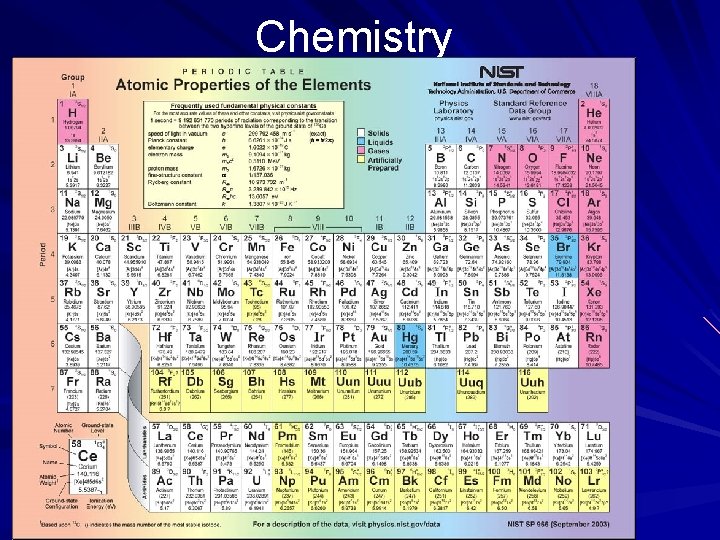
Chemistry Elements and Periodicity In the periodic table, elements are organized according to their atomic and physical properties. The table relates the properties of the elements to their atomic numbers. Elements in the same row (across) have the same number of shells containing a varying number of electrons. Elements in the same column (down) have the same number of electrons in their outermost shell. (page 540)

Chemistry

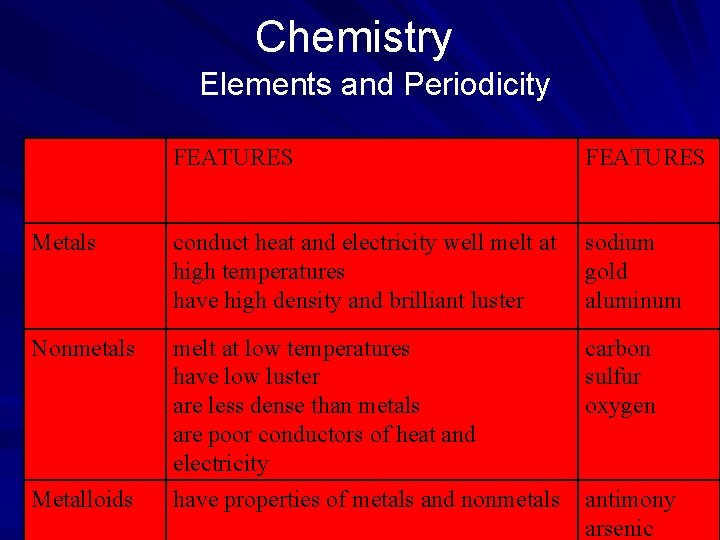
Chemistry Elements and Periodicity In classifying elements according to physical properties, scientists consider color, odor, taste, density, boiling point, solubility (ability to dissolve), malleability (capability of being shaped by beating), and hardness. Out of these properties arose three broad groupings that chemists have used to categorize all of the elements—metals, nonmetals, and metalloids. (page 540)

Chemistry Elements and Periodicity FEATURES Metals conduct heat and electricity well melt at high temperatures have high density and brilliant luster sodium gold aluminum Nonmetals melt at low temperatures have low luster are less dense than metals are poor conductors of heat and electricity carbon sulfur oxygen Metalloids have properties of metals and nonmetals antimony arsenic

Chemistry Elements and Periodicity According to periodic law, as the atomic number increases for elements in a column, similar properties occur regularly and to a greater degree. For example, the metals with the atomic numbers 3, 11, and 19—lithium, sodium, and potassium, respectively—are all chemically active metals. (page 540)

Chemistry Elements and Periodicity In many cases, the greater the atomic number, the higher the degree of certain physical or chemical properties. Whereas the second member of this group, sodium, is chemically active, the fourth member, rubidium, is so highly active that it bursts into flame upon exposure to air. (page 540)

Chemistry EXERCISE 4 Elements and Periodicity (page 542) Directions: Choose the best answer for each of the following questions. 1. The metals copper, silver, and gold are in the same family (column), having atomic numbers of 29, 47, and 79 respectively. According to the principle of periodic law, of the three metals, gold would have the highest degree of which physical property? (1) value (2) rarity (3) volatility (4) malleability (5) scarcity

Chemistry EXERCISE 4 Elements and Periodicity (page 542) 2. Radon is in the same family as helium, neon, argon, krypton, and xenon. Which of the following facts would help you to determine that radon has a greater density than the other elements in the same family? (1) Radon is found in the ground, whereas other elements are not. (2) Radon has a higher atomic number than the other elements in its family. (3) Radon poses potential health problems where great concentrations are found in the ground. (4) Radon is used in many medical treatments that require chemical reactions in the body. (5) Radon is atomically very unstable and is dangerous to use.

Chemistry Elements and Chemical Reactions Each chemical reaction has two components: a reactant and a product. A reactant is the substance or substances that enter into the reaction. The product is the substance or substances that result from the reaction. A chemical reaction may be either a combination reaction, in which two elements or substances are combined, or a decomposition reaction, in which an element or substance is broken down. (page 542)

Chemistry Elements and Chemical Reactions (page 542) A chemical reaction is written in a shorthand called a chemical equation. A chemical formula uses symbols for elements and shows the number of atoms for each element of a substance. For example, the chemical reaction that produces water would be written this way: 2 H 2 + O 2 → 2 H 2 O

Chemistry Elements and Chemical Reactions When you read a chemical equation, the large number tells how many molecules (structures containing more than one atom) are present. When only a single molecule or atom is present, the number 1 is not written. The smaller subscript number tells how many atoms of an element are present in each molecule. (page 543)

Chemistry Elements and Chemical Reactions The equation for water says that two molecules of hydrogen gas (H 2) plus one molecule of oxygen gas (O 2) combine to form two molecules of water (H 2 O). Notice that one molecule of hydrogen gas (H 2) contains two atoms of hydrogen, and one molecule of oxygen gas (O 2) contains two atoms of oxygen. (page 543)

Chemistry Elements and Chemical Reactions (page 543) Each molecule of water (H 2 O) contains two atoms of hydrogen and one atom of oxygen. The chemical reaction in which one atom of carbon unites with two atoms of oxygen to form carbon dioxide would be written this way: C + O 2 → CO 2

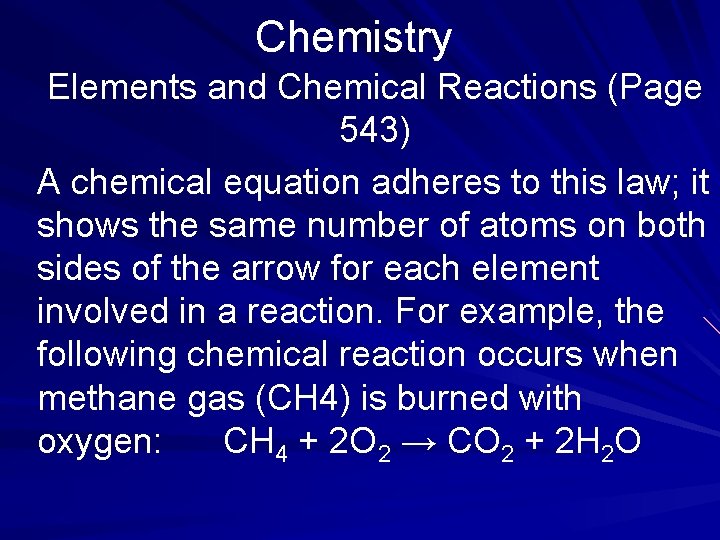
Chemistry Elements and Chemical Reactions This equation says that one molecule of carbon plus one molecule of oxygen (two atoms of oxygen) combine to form one molecule of carbon dioxide (CO 2). All chemical reactions are governed by the Law of Conservation of Matter. This law holds that matter can neither be created nor destroyed in a chemical reaction. (page 543)

Chemistry Elements and Chemical Reactions (Page 543) A chemical equation adheres to this law; it shows the same number of atoms on both sides of the arrow for each element involved in a reaction. For example, the following chemical reaction occurs when methane gas (CH 4) is burned with oxygen: CH 4 + 2 O 2 → CO 2 + 2 H 2 O

Chemistry Elements and Chemical Reactions Methane gas burns with oxygen to form carbon dioxide and water vapor; specifically, one molecule of carbon dioxide and two molecules of water. Notice that the reaction begins with one carbon atom (C) and ends with one carbon atom (C). (page 543)

Chemistry Elements and Chemical Reactions The reaction begins with four hydrogen atoms (H 4) and ends with four hydrogen atoms (2 H 2 or 2 X 2 = 4). The reaction begins with four oxygen atoms (2 O 2 = 2 X 2 = 4) and ends with 4 oxygen atoms (O 2 + 2 O = 4). When the number of atoms of each element is equal on both sides of the equation, we say that the equation is balanced. (page 543)

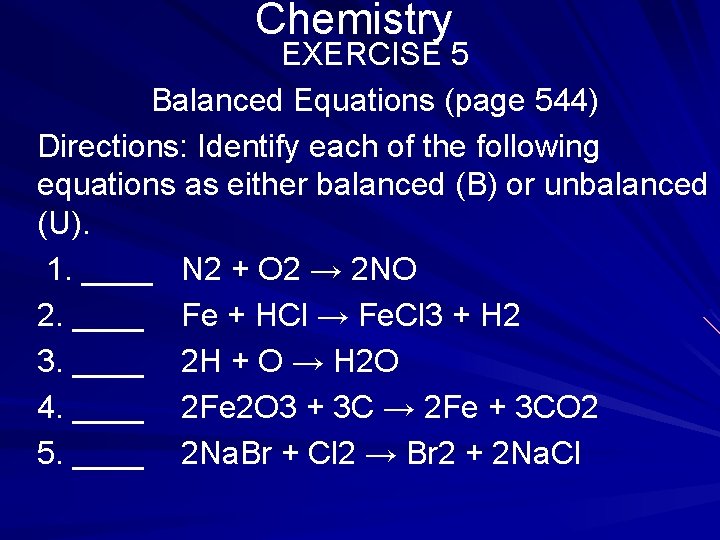
Chemistry EXERCISE 5 Balanced Equations (page 544) Directions: Identify each of the following equations as either balanced (B) or unbalanced (U). 1. ____ N 2 + O 2 → 2 NO 2. ____ Fe + HCl → Fe. Cl 3 + H 2 3. ____ 2 H + O → H 2 O 4. ____ 2 Fe 2 O 3 + 3 C → 2 Fe + 3 CO 2 5. ____ 2 Na. Br + Cl 2 → Br 2 + 2 Na. Cl

Chemistry EXERCISE 6 Chemical Reactions (page 544) Directions: Choose the best answer for each of the following questions. 1. The chemical reaction that gives soda pop (a carbonated beverage) its fizz results from dissolving a molecule of carbon dioxide into a molecule of water. Which of the following represents the chemical equation for the process? (1) CO 3 + H 2 O → H 2 CO 4 (2) CO 2 + H 2 O → H 2 CO 3 (3) CO + H 2 O → H 2 CO 2 (4) CO 2 + H 2 O → H 2 CO 2 (5) CO + 2 H 2 O → H 4 CO 2

Chemistry EXERCISE 6 (page 545) Chemical Reactions 2. What is the relation of reactants to products of a chemical reaction? (1) They always double in mass. (2) They must always balance. (3) They never equal each other in mass. (4) They always need a catalyst. (5) They must triple themselves to balance.

Chemistry EXERCISE 6 Chemical Reactions Question 3 refers to the following passage. A physical change is a change that does not produce a new substance. For example, when you saw wood or dissolve salt in water you are not changing the chemical composition of the substances. However, a new substance is formed when a chemical change takes place. The result is a change in the chemical composition of a substance. Some common chemical changes include the burning of wood and the rusting of metal on a car. (page 545)

Chemistry EXERCISE 6 Chemical Reactions Question 3 refers to the following passage. In an experiment concerning physical and chemical changes, you add 10 g of copper sulfate to 100 ml of water. You heat the solution over a low flame and stir. After the solution cools, you place a piece of aluminum foil in the coper sulfate solution. After 24 hours, the solution has changed from deep blue to a very light blue, and the aluminum has acquired a deep copper coating. (page 545)

Chemistry EXERCISE 6 Chemical Reactions (page 545) 3. Which of the following pieces of information would you need to see at the end of the experiment to prove that a chemical change or a new substance had occurred? (1) whether the copper sulfate solution had been heated (2) whether the solution had been stirred (3) whether twenty-four hours had passed (4) whether the aluminum had acquired a copper coat (5) whether the aluminum was breakable

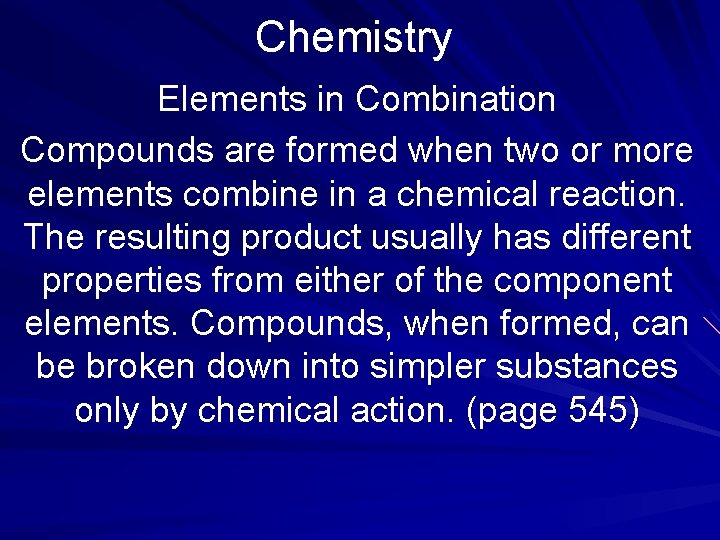
Chemistry Elements in Combination Compounds are formed when two or more elements combine in a chemical reaction. The resulting product usually has different properties from either of the component elements. Compounds, when formed, can be broken down into simpler substances only by chemical action. (page 545)

Chemistry Elements in Combination For example, water, the most commonly known compound, is composed of two atoms of hydrogen and one atom of oxygen. When water is subjected to extreme temperatures, it can be reduced to its component elements—hydrogen and oxygen—and its liquid characteristic is lost. (page 545)

Chemistry Elements in Combination Mixtures are substances that are formed when two or more elements or compounds are mixed in different proportions. The resulting product retains the properties of the combining elements. In most mixtures the combining ingredients can be separated easily. (page 545)

Chemistry Elements in Combination For example, gunpowder is a mixture of charcoal (a form of carbon), sulfur, and potassium nitrate (a compound of potassium and nitrogen). When mixed, the three ingredients form gunpowder, a highly explosive substance. These three ingredients can be identified by their different colors in this mixture. (page 545)

Chemistry Elements in Combination A solution is a mixture formed when a solid, liquid, or gaseous substance is dissolved in a liquid. The substance that is dissolved into the liquid is called the solute. The liquid in which the substance is dissolved is called the solvent. (page 546)

Chemistry Elements in Combination One of the characteristics that distinguishes a solution from a mixture is that a solution is homogeneous—the same throughout. An aqueous solution features water as the solvent. A tincture, such as the antiseptic tincture of iodine, has alcohol as the solvent. Sometimes a solution is formed when a substance is dissolved in a gas or solid. (page 546)

Chemistry Elements in Combination When metals are combined in varying proportions, they often form alloys. In an alloy, each metal dissolves into the other at high temperatures. Common examples of alloys are brass (copper and zinc), bronze (copper, tin, and other elements), and steel (iron, carbon, and other elements). (page 546)

Chemistry Elements in Combination An amalgam is formed when a metal is dissolved into mercury, a liquid metal. Amalgams are used chiefly in making tooth cements and are referred to as silver fillings (although more people today choose the porcelain or tooth-colored fillings). (page 546)

Chemistry EXERCISE 7 Elements in Combination (page 546) Directions: Read the definitions below of five kinds of substances known to scientists. Then choose the best answer to the questions that follow. compound a substance that is composed of two or more elements, in specific proportions, but has properties different from the combining elements

Chemistry EXERCISE 7 Elements in Combination (page 546) mixture a substance that is composed of two or more elements or other substances but that keeps the properties of the combining ingredients solution a homogeneous substance formed by dissolving a solid, liquid, or gas into a liquid

Chemistry EXERCISE 7 Elements in Combination (page 546) alloy a substance formed by the combination of metals in which one metal dissolves into another at high temperatures amalgam an alloy that includes mercury and that is usually soft and may be liquid

Chemistry EXERCISE 7 Elements in Combination (page 546) 1. Table salt—sodium chloride, one of the most common substances occurring in nature—is best classified as which of the following? (1) a compound (2) a mixture (3) a solution (4) an alloy (5) an amalgam

Chemistry EXERCISE 7 Elements in Combination (page 547) 2. Air is composed of nitrogen (78 percent), oxygen (21 percent), argon (0. 93 percent), carbon dioxide (0. 03 percent), and other gases (0. 04 percent). How may air be best described? (1) a compound (2) a mixture (3) a solution (4) an alloy (5) an amalgam

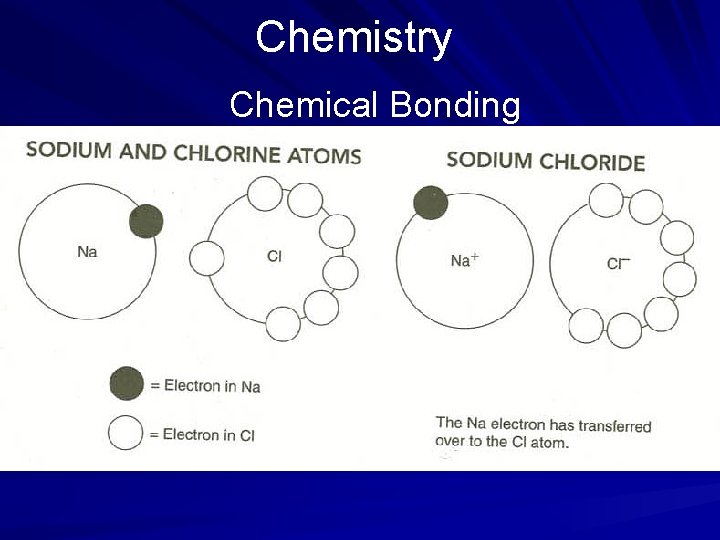
Chemistry Chemical Bonding When compounds are made, a bond is formed between two or more elements. A bond is a force that holds together two atoms, two ions (electrically charged particles), two molecules, or a combination of these. Bonding may result from either the transfer or the sharing of electrons between atoms. (page 547)

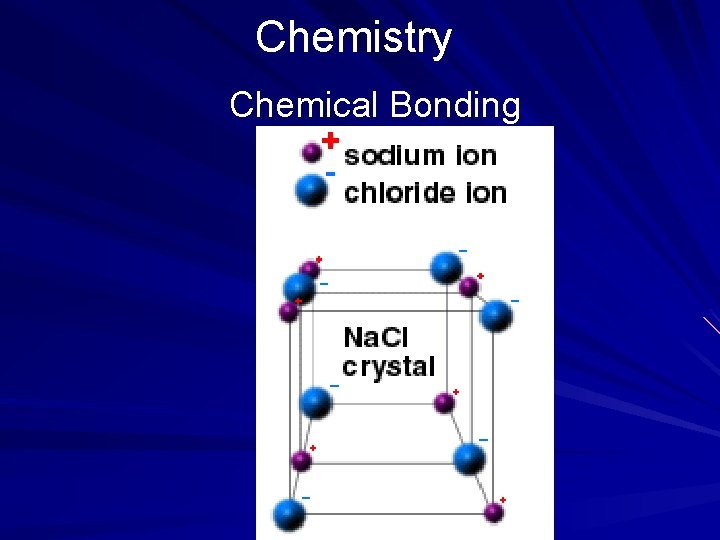
Chemistry Chemical Bonding When electrons are transferred from one atom to another, an ionic bond is formed. In the following example, an ionic bond is formed when an electron from a sodium atom is transferred to the outermost shell of a chlorine atom. The result is the common compound table salt. (page 547)

Chemistry Chemical Bonding

Chemistry Chemical Bonding

Chemistry Chemical Bonding In the preceding example, both sodium and chlorine are electrically neutral (have no charge); however, when the sodium atom loses its electron, it becomes positively charged. Opposite charges attract, forming a bond. Ionic compounds such as salt typically have high melting and boiling points, are flammable, conduct electricity when dissolved in water, and exist as solids at room temperatures. (page 547)

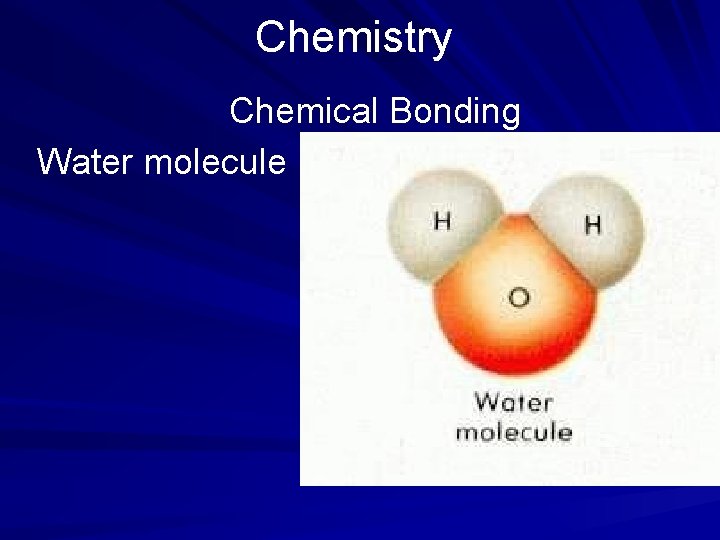
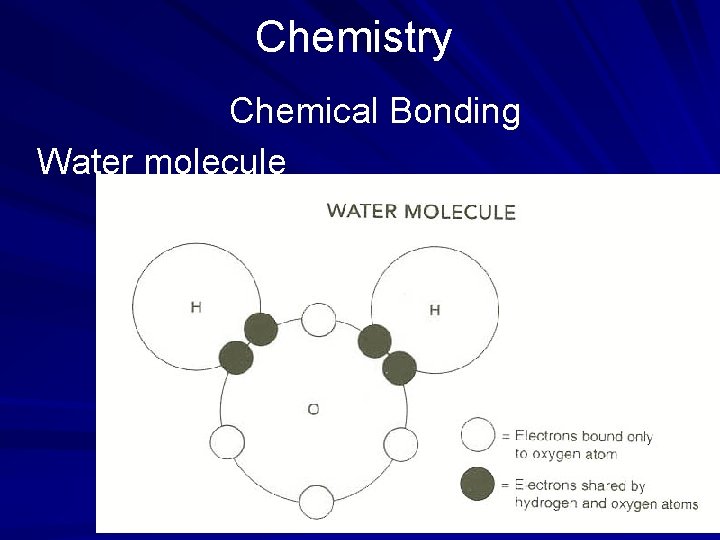
Chemistry Chemical Bonding When two or more atoms of different elements share electrons to form a molecule, a covalent bond is formed. A covalent bond is formed when two atoms of hydrogen are bonded to one atom of oxygen to form the compound water. (page 547)

Chemistry Chemical Bonding Water molecule

Chemistry Chemical Bonding Water molecule

Chemistry Chemical Bonding In covalent bonding, the outermost shell of the element with the greatest number of electrons is filled to capacity at eight electrons. Once the combining element achieves eight electrons in its outermost ring, it cannot combine with another element. Covalent compounds such as water typically have low melting and boiling points, are nonflammable, have poor conductivity, and exist as gases and liquids. (page 548)

Chemistry Chemical Bonding Polymers

Chemistry Chemical Bonding Polymers have been around since the turn of the 20 th century. At that time chemists found that the waste products from organic compounds of phenol and formaldehyde could be treated with heat and high pressure. The resulting material is very hard and is used to make billiard balls and telephones as well as handles on pots and pans. (page 548)

Chemistry Chemical Bonding More research with these kinds of organic compounds led to the invention of nylon 66 by two chemists from the Dupont company. Vulcanized rubber that is used to make automobile tires is also the product of experimenting with the bonding of these types of polymer chains. (page 548)

Chemistry Chemical Bonding Another type of synthetic polymer is polyester which is used in the making of many types of fabric. Another example of wearable polymers is acrylic. Acrylic feels like wool but is less expensive and can be machine washed. (page 549)

Chemistry Chemical Bonding The fabric industry cautions that heat from a fire breaks the bonds, and the fragments react with oxygen to continue the burning reaction, possibly adhering to the skin. Warning labels are put on the garments; some fabrics, especially for sleepwear, are treated with flame-retardant material. (page 549)

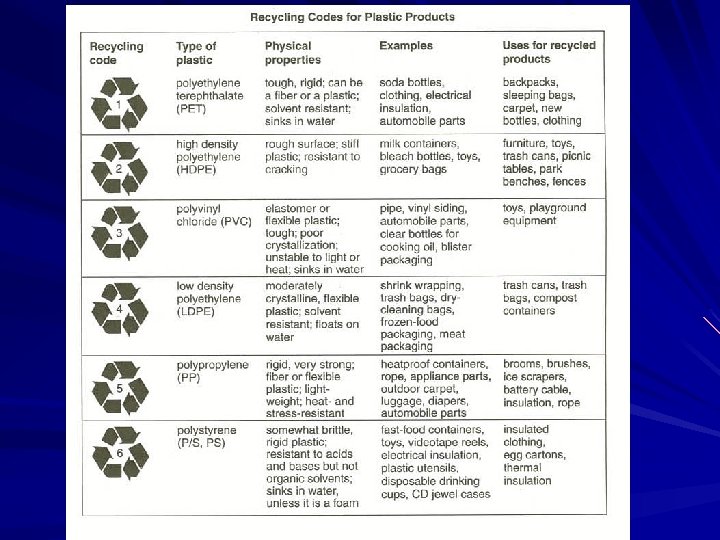
Chemistry Chemical Bonding Plastics are another chemical invention of bonding. The variety of chemical bonds allows some plastics, such as PET, to be tough and solvent. This plastic, which has a recycling code of 1, can be found in soda bottles and recycled into new bottles or carpeting or sleeping bags. Another type of plastic (PVC) is a tough, flexible plastic used in pipes or vinyl siding and can be recycled into toys and playground equipment. (page 549)

Chemistry Chemical Bonding

Chemistry EXERCISE 8 Chemical Bonding (page 550) Directions: Choose the best answer for each of the following questions. 1. In ionic bonding, how are atoms are held together? (1) by sharing electrons (2) by transferring electrons (3) by chemical attraction (4) by temperature (5) by cohesion

Chemistry EXERCISE 8 Chemical Bonding (page 550) 2. In covalent bonding, how are atoms held together? (1) by sharing electrons (2) by transferring electrons (3) by chemical attraction (4) by temperature (5) by cohesion

Chemistry EXERCISE 8 Chemical Bonding (page 550) 3. Use the Recycling Code Chart to identify the correct code from 1 to 6 for the following items. ____ shrink wrap, trash bags, and meat packaging ____ milk containers, toys and bleach bottles ____ diapers, luggage, and appliance parts ____ fast food containers, VCR cassettes and utensils ____ pipes, automobile parts, and clear bottles for cooking oil

Chemistry Acids, Bases, and Salts Many compounds that result from ionic and covalent bonding are categorized as acids or bases. An acid is a covalent compound that produces hydrogen ions when dissolved in water. Acids have a sour taste. Common acids are acetic acid (the main component of vinegar), citric acid (found in citrus fruits), lactic acid (found in milk), and hydrochloric acid, a component of stomach acid used in digestion. (page 550)

Chemistry Acids, Bases, and Salts A base is a compound that forms hydroxide ions when dissolved in water. Bases are able to take a proton from an acid or to give up an unshared pair of electrons to an acid. Bases are described as alkaline because they dissolve in water and have a slippery feel. Many hydroxides are bases. Household cleaning agents such as ammonia, borax, lye, and detergents are common examples of bases. (page 550)

Chemistry Acids, Bases, and Salts When an acid combines with a base, a salt is formed and water released because the metal found in the base replaces the hydrogen contained in the acid. Inorganic acids, bases, and inorganic salts can conduct electricity when dissolved in water. Chemists apply the litmus test to a substance to determine whether it is an acid or a base. An acid turns blue litmus paper red, and a base turns red litmus paper blue. (page 551)

Chemistry Acids, Bases, and Salts Litmus paper test

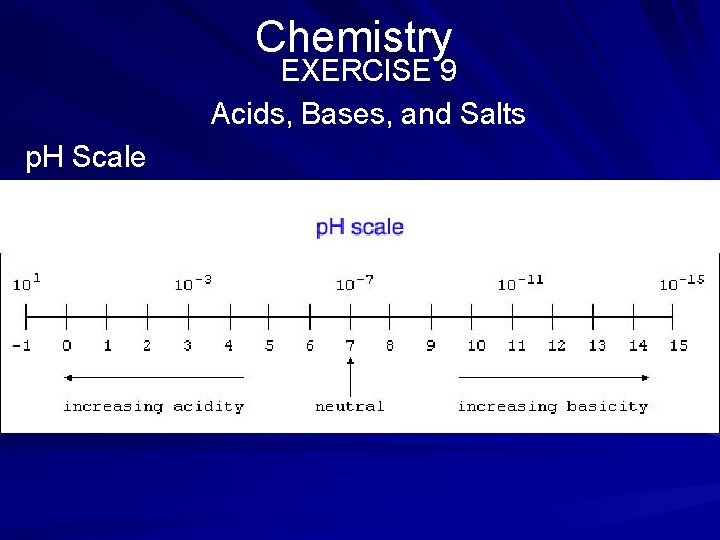
Chemistry EXERCISE 9 Acids, Bases, and Salts Directions: Read the explanation and scale below. Choose the best answer for each question that follows. The designation p. H (potential for hydrogen-ion formation) is a value by which certain substances are classified according to acidity or alkalinity. The p. H scale ranges from 0 to 14, with the value 7 representing neutrality. The p. H scale is illustrated below. (page 551)

Chemistry EXERCISE 9 Acids, Bases, and Salts p. H Scale

Chemistry EXERCISE 9 Acids, Bases, and Salts (page 551) 1. According to the p. H scale, between which numbers would acetic acid, a very mild acid, most likely be found? (1) 7 and 8 (2) 0 and 1 (3) 2 and 3 (4) 4 and 5 (5) 10 and 11

Chemistry EXERCISE 9 Acids, Bases, and Salts (page 551) 2. According to the p. H scale, where would ordinary tap water be found? (1) between 0 and 1 (2) between 3 and 4 (3) exactly at 7 (4) exactly at 1 (5) between 5 and 6

Chemistry EXERCISE 9 Acids, Bases, and Salts (page 551) 3. Which of the following could be used to prove that a substance is an acid? (1) The substance has a p. H above 7. (2) The substance has a slippery feel. (3) It neutralizes a base to form a salt and water. (4) When mixed with water, a solid would form. (5) The liquid turns red when mixed with water.

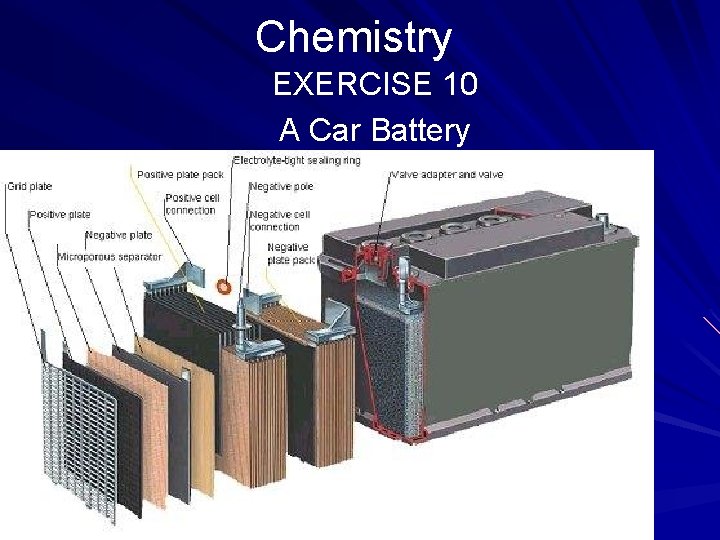
Chemistry EXERCISE 10 A Car Battery Directions: Read the passage below and answer the questions that follow. Acids, bases, and inorganic salts (salts obtained from nonliving things) are effective conductors of electricity. The common car battery demonstrates an electric current generated by the chemical action between an acid and a metal. (page 552)

Chemistry EXERCISE 10 A Car Battery

Chemistry EXERCISE 10 A Car Battery In a car battery pure lead (the negative post) and lead dioxide (the positive post) are submerged in sulfuric acid (the conductor). Distilled water is added periodically to maintain the proper level of sulfuric acid. The pure lead loses two electrons when it reacts with the sulfuric acid—the acid changes the lead to lead dioxide. (page 552)

Chemistry EXERCISE 10 A Car Battery At the same time, the positive post containing lead dioxide gains two electrons and changes the sulfuric acid in which it is submerged into lead sulfate (a salt) and water. The current that makes the car start results from the flow of electrons from the lead dioxide to the lead through the sulfuric acid to the starter switch, all of which makes a complete circuit. (page 552)

Chemistry EXERCISE 10 A Car Battery (page 553) 1. An electrolyte is an inorganic compound that will conduct an electric current when dissolved in water. What is an electrolyte in the preceding example? (1) lead dioxide (2) sulfuric acid (3) distilled water (4) carbon particulates (5) carbonic acid

Chemistry EXERCISE 10 A Car Battery (page 553) 2. A substance is oxidized when it loses electrons. In the preceding example, which of the following compounds is oxidized? (1) lead (2) lead dioxide (3) lead sulfate (4) water (5) sulfuric acid

Chemistry EXERCISE 10 A Car Battery (page 553) 3. The substances that oxidize and reduce other substances are called oxidizing agents and reducing agents. According to the reading, in which item are the oxidizing and reducing agents in the correct order? (1) lead dioxide and water (2) sulfuric acid and lead dioxide (3) lead and sulfuric acid (4) lead sulfate and lead dioxide (5) sulfuric acid and water

Chemistry EXERCISE 10 A Car Battery (page 553) 4. Which of the following can you conclude to be true when a battery is discharged and can no longer generate a current? (1) The sulfuric acid can no longer oxidize the lead. (2) The lead dioxide can no longer reduce the sulfuric acid. (3) The battery has run out of sulfuric acid. (4) The amount of water is too low to generate power. (5) The metal casing has corroded.

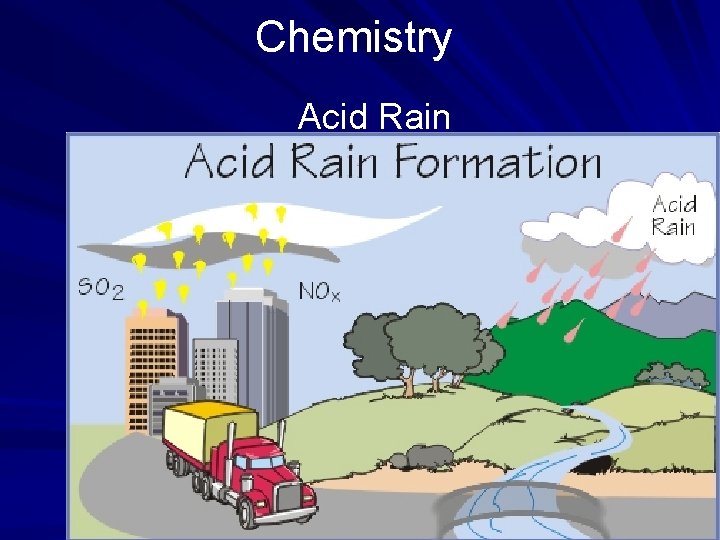
Chemistry Acid Rain A common example of acid's ability to corrode is seen in the increasing acidity of rainwater. As pollutants such as sulfur and carbon from factories enter the water cycle, new compounds are created. Sulfuric acid in weak concentration as well as carbonic acid come down in the form of precipitation. (page 553)

Chemistry Acid Rain When these acids come into contact with stone statues or metalwork, corrosion occurs. Old gravestones, marble facades on buildings, and inscriptions that can no longer be read exemplify the disintegration that occurs gradually every time it rains. (page 553)

Chemistry Acid Rain

Chemistry Acid Rain

Chemistry Acids in the Human Body A variety of acids is found in the human body. Your body actually does a remarkable job in controlling its own p. H balance. Your blood, for example, needs to remain within the range of 7. 35 and 7. 45. If the p. H of your blood is more acidic, you suffer acidosis. (page 553)

Chemistry Acids in the Human Body If the p. H is above 7. 45, you are said to have alkalosis. The body fights the change in p. H by natural chemical balancers called buffers. A buffer solution has a weak acid and its conjugate base in equal amounts. The liquid portion of blood is an example of a buffer solution. (page 553)

Chemistry Acids in the Human Body In some cases, the body may generate too much acid. This is often the case when someone experiences heartburn, which can be temporarily relieved by taking an antacid to neutralize the excess stomach acid. (page 553)

Chemistry Reaction Rate, Catalysts, and Equilibrium Chemical reactions occur at different rates determined by the conditions under which the reactions take place. Sugar dissolves more quickly in hot water than in cold. White phosphorus bursts into flame when exposed to the air. Reactions such as these may be speeded up or slowed down when another substance is introduced. (page 554)

Chemistry Reaction Rate, Catalysts, and Equilibrium A catalyst is a substance that increases the rate of a chemical reaction but itself remains chemically unchanged. Some catalysts have a negative effect. A negative catalyst slows down the chemical reaction. Negative catalysts, such as the chemicals used in undercoating a car to retard the rusting process, are often inhibitors. (page 554)

Chemistry Reaction Rate, Catalysts, and Equilibrium A given set of reactants may react to form more than one product. Often, the byproducts react to form the original reactants. When the rate of forward reaction balances the rate of reverse reaction, chemical equilibrium occurs. (page 554)

Chemistry Reaction Rate, Catalysts, and Equilibrium For example, the carbon monoxide in car exhaust systems enters the atmosphere and reacts with oxygen to form carbon dioxide. (page 554)

Chemistry Reaction Rate, Catalysts, and Equilibrium The carbon dioxide is broken down by sunlight into the original reactant—carbon monoxide. This reaction represents chemical equilibrium because the reaction reverses itself. Chemical reactions such as these create a cycle. (page 554)

Chemistry EXERCISE 11 Reaction Rate, Catalysts, and Equilibrium (page 554) Directions: Choose the best answers for the following questions. 1. Lipase is an enzyme produced by the liver that helps in the digestion of fats by speeding up the rate at which lipids (fats) are changed into fatty acids and glycerol. According to this description, what can we conclude that an enzyme is? (1) a product in a chemical reaction (2) a negative catalyst (3) a biological catalyst (4) a temporary product (5) a by-product that is unstable

Chemistry EXERCISE 11 Reaction Rate, Catalysts, and Equilibrium (page 554) 2. Which of the following processes illustrates chemical equilibrium? (1) bonding (2) photosynthesis (3) respiration (4) oxidation (5) organic synthesis
 Section 1 composition of matter
Section 1 composition of matter What is white matter made of
What is white matter made of Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Median and lateral apertures
Median and lateral apertures Composition of matter section 1
Composition of matter section 1 Gray matter and white matter
Gray matter and white matter Ncl. caudatus
Ncl. caudatus Ecological succession
Ecological succession Definition of substance
Definition of substance Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 chemistry study guide
Chapter 10 chemistry study guide Examples of matter in chemistry
Examples of matter in chemistry Flowchart of matter
Flowchart of matter 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Chemistry matter and change chapter 2 answer key
Chemistry matter and change chapter 2 answer key Flowchart undissolved solids
Flowchart undissolved solids Graphic organizer about matter
Graphic organizer about matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Foods that are heterogeneous mixtures
Foods that are heterogeneous mixtures The study of composition structure and properties of matter
The study of composition structure and properties of matter Study of matter
Study of matter Chapter 12 states of matter study guide
Chapter 12 states of matter study guide Whats the study of matter and energy
Whats the study of matter and energy Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Chemistry unit 2 study guide answer key
Chemistry unit 2 study guide answer key Chapter 11 chapter assessment stoichiometry answer key
Chapter 11 chapter assessment stoichiometry answer key Chemistry semester 1 exam review answers
Chemistry semester 1 exam review answers If a laboratory fire erupts, immediately
If a laboratory fire erupts, immediately Vce chemistry research investigation example
Vce chemistry research investigation example What is case series
What is case series Retrospective cohort study vs prospective cohort study
Retrospective cohort study vs prospective cohort study Critical examination in method study
Critical examination in method study Marty lobdell study less study smart
Marty lobdell study less study smart Phytogeographical region of india
Phytogeographical region of india Differentiate between time study and motion study
Differentiate between time study and motion study Differentiate between time study and motion study
Differentiate between time study and motion study Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worms-breton
Tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? * Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Bảng số nguyên tố
Bảng số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Why business models matter
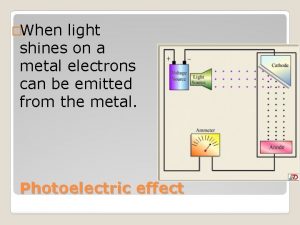
Why business models matter Does it matter what type of light shines on the metal?
Does it matter what type of light shines on the metal? Greek word of ekonomiks
Greek word of ekonomiks Carries energy through matter
Carries energy through matter Wave transfer matter
Wave transfer matter Flow chart of matter
Flow chart of matter State of matter
State of matter Formal versus informal communication
Formal versus informal communication Properties of liquid in matter
Properties of liquid in matter Holy eucharist form
Holy eucharist form Closely packed in an orderly manner
Closely packed in an orderly manner The mole measuring matter
The mole measuring matter Define the subject matter of psychology
Define the subject matter of psychology What is the particle theory of matter
What is the particle theory of matter Four phases of matter
Four phases of matter Four states of matter
Four states of matter States of matter
States of matter Thermal energy states of matter
Thermal energy states of matter States of matter graph
States of matter graph Sixth state of matter
Sixth state of matter States of matter
States of matter Plasma state of matter examples
Plasma state of matter examples Changing states of matter
Changing states of matter Phet states of matter basics
Phet states of matter basics 5 states of matter
5 states of matter 4 phases of matter
4 phases of matter Soil is a mixture of weathered rock and ________.
Soil is a mixture of weathered rock and ________. Subject matter sociology
Subject matter sociology Say/mean/matter
Say/mean/matter Say mean matter
Say mean matter 3 states of matter venn diagram
3 states of matter venn diagram The kinetic theory of matter states that
The kinetic theory of matter states that Rankings: what are they and do they matter?
Rankings: what are they and do they matter? Mixture graphic organizer
Mixture graphic organizer Properties of matter vocabulary
Properties of matter vocabulary Kinetic theory of matter definition
Kinetic theory of matter definition Properties of matter concept map
Properties of matter concept map Objectives of properties of matter
Objectives of properties of matter General property of matter
General property of matter Classification and properties of matter
Classification and properties of matter Properties and changes of matter worksheet
Properties and changes of matter worksheet Particle model of matter exam questions
Particle model of matter exam questions Mechanical waves and electromagnetic waves similarities
Mechanical waves and electromagnetic waves similarities Chemical property definition
Chemical property definition Chemical properties
Chemical properties 5 phases of matter
5 phases of matter What is the particulate model of matter
What is the particulate model of matter Classifying matter quiz
Classifying matter quiz Quarks
Quarks Other people matter mindset
Other people matter mindset What is inorganic matter
What is inorganic matter Convex mirror matter
Convex mirror matter Rhythmic movement that carries energy through matter
Rhythmic movement that carries energy through matter Say mean matter chart
Say mean matter chart Horn of grey matter
Horn of grey matter Natural sciences and technology
Natural sciences and technology Natural science grade 7 term 3 notes
Natural science grade 7 term 3 notes Ns grade 6 term 2
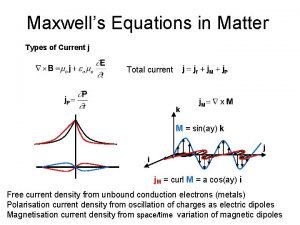
Ns grade 6 term 2 Maxwell equation in matter
Maxwell equation in matter Matter antimatter asymmetry
Matter antimatter asymmetry Composition uniform
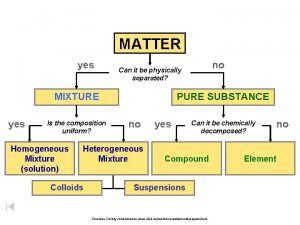
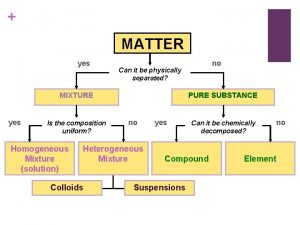
Composition uniform Matter can it be physically separated
Matter can it be physically separated What are matter waves
What are matter waves Matter chart
Matter chart Pepsi particles candy
Pepsi particles candy Examples of chemical properties of matter
Examples of chemical properties of matter Quilantan entry evidence
Quilantan entry evidence