Chemistry the science of matter its properties structure



























- Slides: 40

Chemistry: the science of matter; its properties, structure, composition, behaviour, reactions, interactions and changes Properties of Matter: Anything with mass that takes up space

Exploring the Nature of Matter All matter exists in one of three states: � Solid: Definite shape and volume � Liquid: definite volume not shape � Gas: Indefinite volume and shape

The Particle Theory of Matter � Matter and its behavior can be explained using a scientific model called the particle theory of matter

The Particle Theory of Matter Five Postulates (Assumptions): � 1. All matter is made of small particles. - particle � 2. All particles of one substance are identical. - water (liquid, ice, or steam) particles - oxygen particles

The Particle Theory of Matter 3. The particles of matter attract one another. -in solids, strong forces keep particles together -in liquids the forces are weaker -in gases, the forces are weakest; the particles are further apart

The Particle Theory of Matter 4. The particles of matter are constantly in motion. solids vibrate liquids - more motion gases – most motion

The Particle Theory of Matter 5. Particles at a higher temperature move faster on average than particles at a lower temperature.

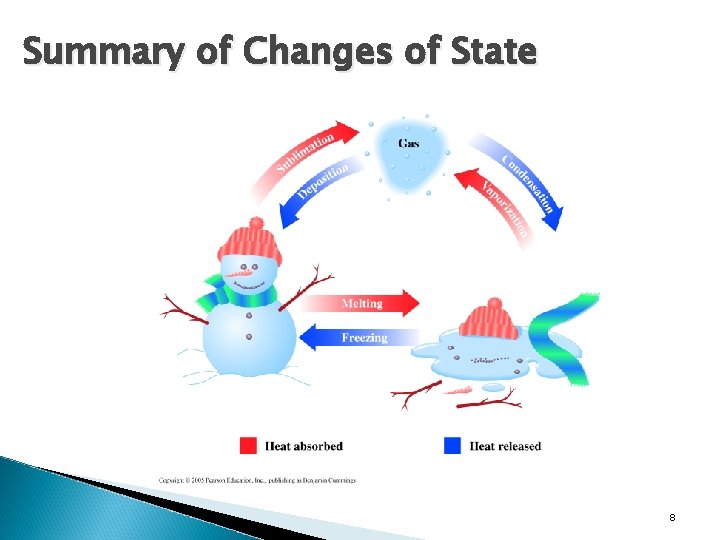
Summary of Changes of State 8

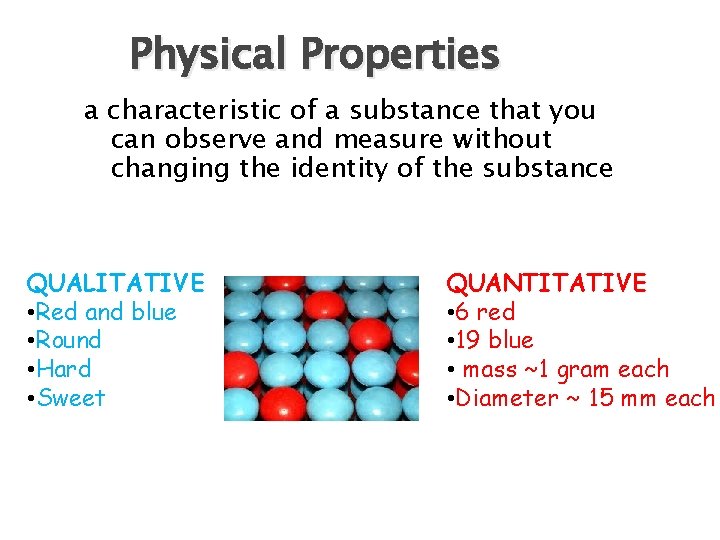
Physical Properties a characteristic of a substance that you can observe and measure without changing the identity of the substance QUALITATIVE • Red and blue • Round • Hard • Sweet QUANTITATIVE • 6 red • 19 blue • mass ~1 gram each • Diameter ~ 15 mm each

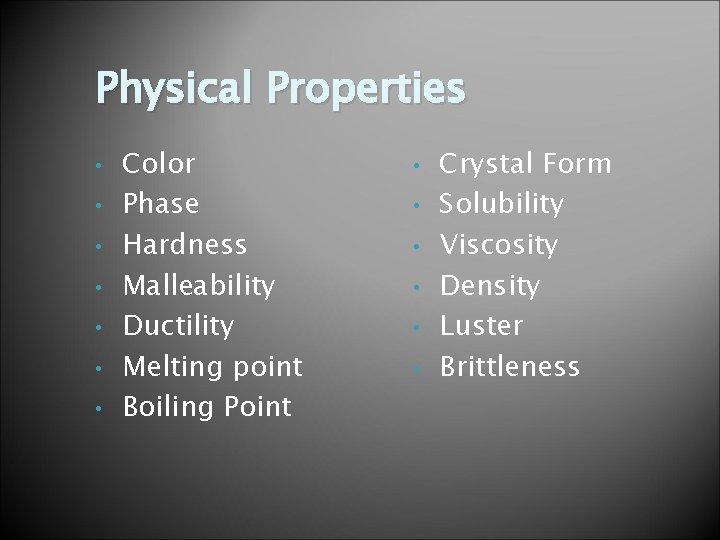
Physical Properties • • Color Phase Hardness Malleability Ductility Melting point Boiling Point • • • Crystal Form Solubility Viscosity Density Luster Brittleness

Hardness • How difficult it is to scratch a substance

Malleability • Most metals are malleable - they can be bent or hammered into different shapes

• Brittleness Substances that are non-malleable are also called “brittle” MICA

Ductility • Ability of a material to be stretched into a wire without breaking

Solubility � Ability of a substance to dissolve in another SOLUBLE INSOLUBLE

Viscosity � How thick or hard to pour a liquid is

Density � Mass per unit of volume

Which is heavier? 1 Kg of Feathers or 1 Kg of Lead?

�A Physical change in which the composition of the substance remains the same and no new substances are produced

Physical Property or Change? �Cutting paper? Physical change

Physical Property or Change? �Ice melting? Physical change

Physical Property or Change? • Elasticity? Physical property

Chemical Properties Describe how a substance behaves as it changes into a new substance • E. g. - Flammable � - Corrosivity � - Reactivity •

Chemical Change A change in which new chemically different substances are produced • E. g. - wood burning in O 2 to produce C 02 + carbon ash + H 2 O � - reaction of baking soda with water to produce C 02 • �

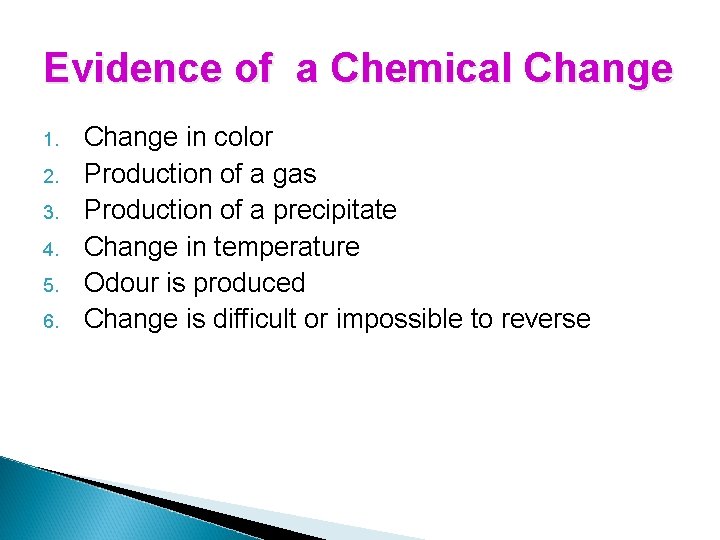
Evidence of a Chemical Change 1. 2. 3. 4. 5. 6. Change in color Production of a gas Production of a precipitate Change in temperature Odour is produced Change is difficult or impossible to reverse

Chemical or Physical Change? �Toast burning? Chemical

Physical Property or Change? �Lava cooling? Physical change

Chemical or Physical Change? � Rocket fuel burning? Chemical

Chemical or Physical Change? �Metal rusting? Chemical

Physical Property or Change? �Sawing wood? Physical change

Chemical or Physical Change? �Candle Chemical burning?

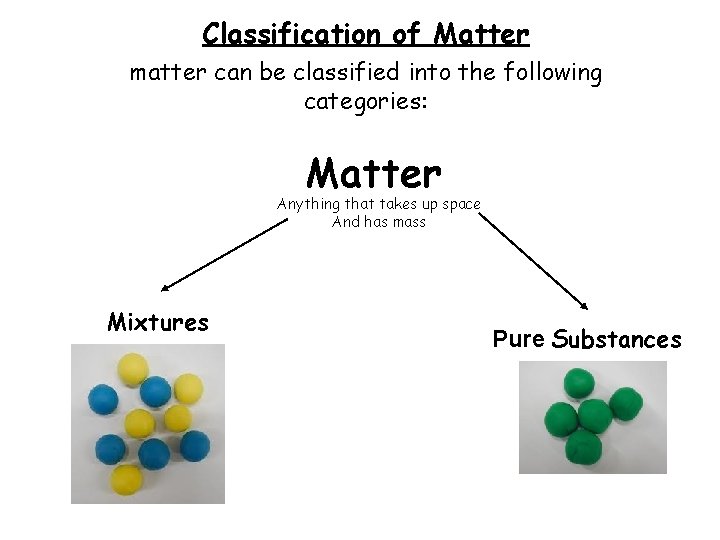
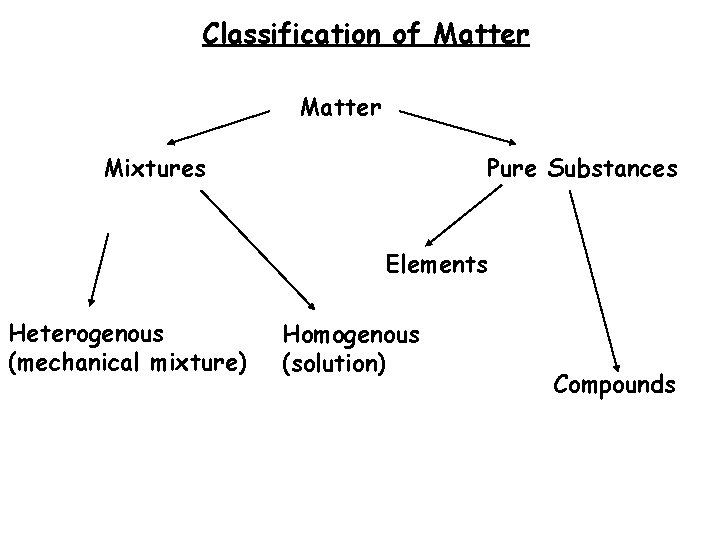
Classification of Matter matter can be classified into the following categories: Matter Anything that takes up space And has mass Mixtures Pure Substances

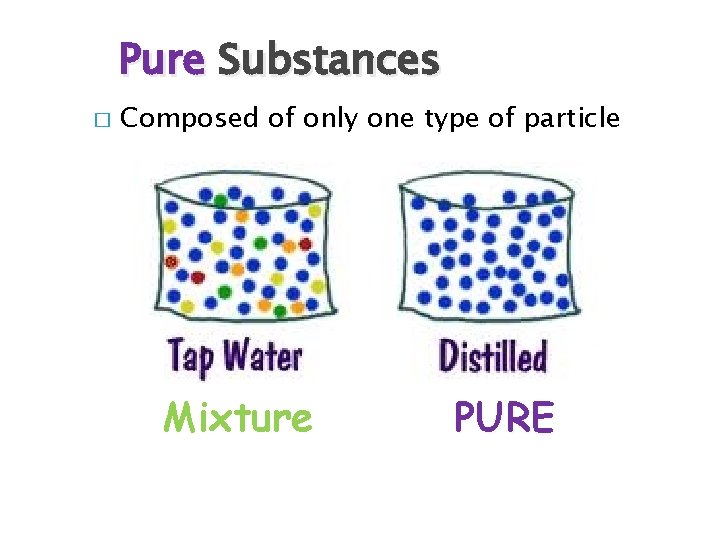
Pure Substances � Composed of only one type of particle Mixture PURE

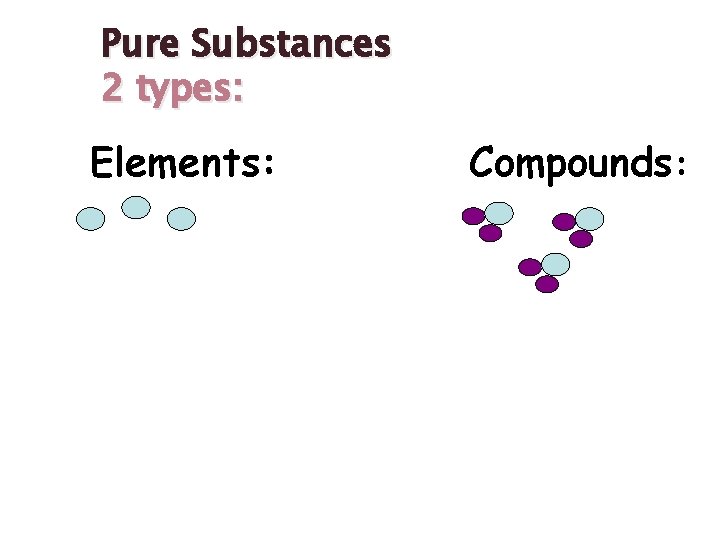
Pure Substances 2 types: Elements: Compounds:

Pure Substances 2 types: ELEMENTS: -Only one kind of particle called an atom - Cannot be broken down into simpler form - Listed in the Periodic Table - Written as a symbol, E. g. Oxygen = O

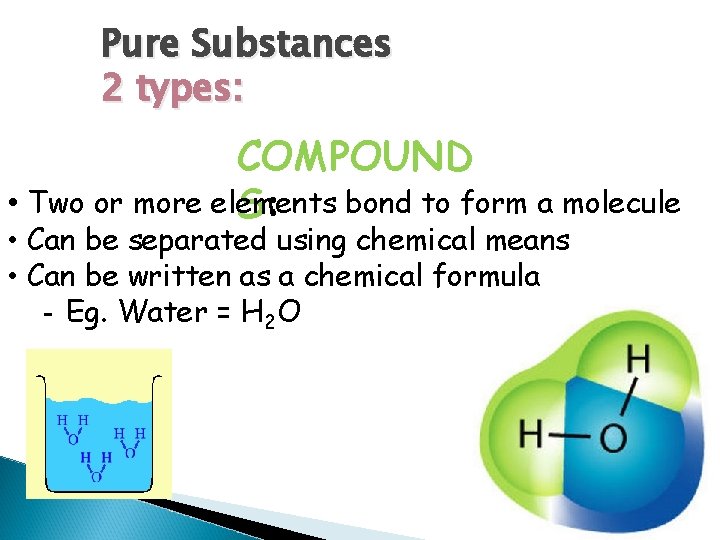
Pure Substances 2 types: COMPOUND • Two or more elements bond to form a molecule S: • Can be separated using chemical means • Can be written as a chemical formula - Eg. Water = H 2 O

• • Mixtures Composed of 2 or more pure substances Can be separated using physical means � 2 Homogenous (solution) types: Heterogenous (mechanical mixture)

Mixtures Homogenous (solution) - Only 1 visible phase - Uniformly mixed together - E. . Kool-Aid, tea, salt water Heterogenous (mechanical mixture) - Two or more visible phases - E. g. Cereal, oil and vinegar, vegetable soup

Mixture or Pure Substance? • • • Helium Salad dressing Nuts and bolts Sodium chloride Gatorade Jello • • • Pure -element Mixture-homogenous/heterogeneous Mixture- heterogeneous Pure- compound Mixture- homogenous

Classification of Matter Mixtures Pure Substances Elements Heterogenous (mechanical mixture) Homogenous (solution) Compounds