Chemistry SOL Review Day 2 SOL 3 UNIT



- Slides: 19

Chemistry SOL Review Day 2 SOL 3

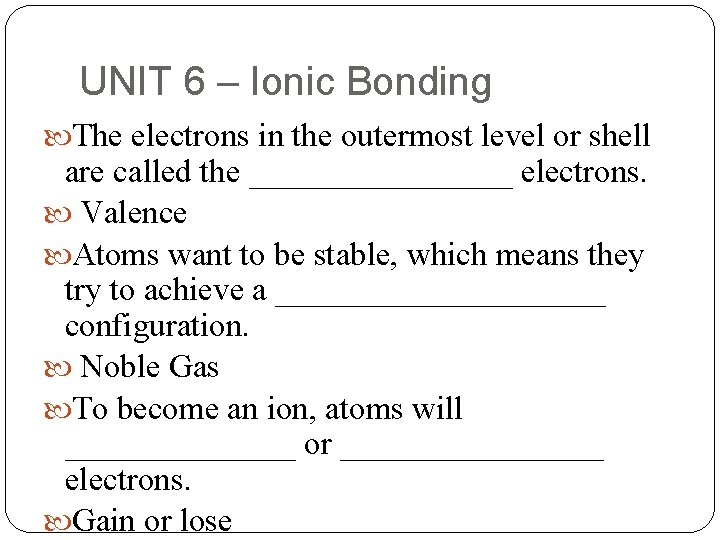
UNIT 6 – Ionic Bonding The electrons in the outermost level or shell are called the ________ electrons. Valence Atoms want to be stable, which means they try to achieve a __________ configuration. Noble Gas To become an ion, atoms will _______ or ________ electrons. Gain or lose

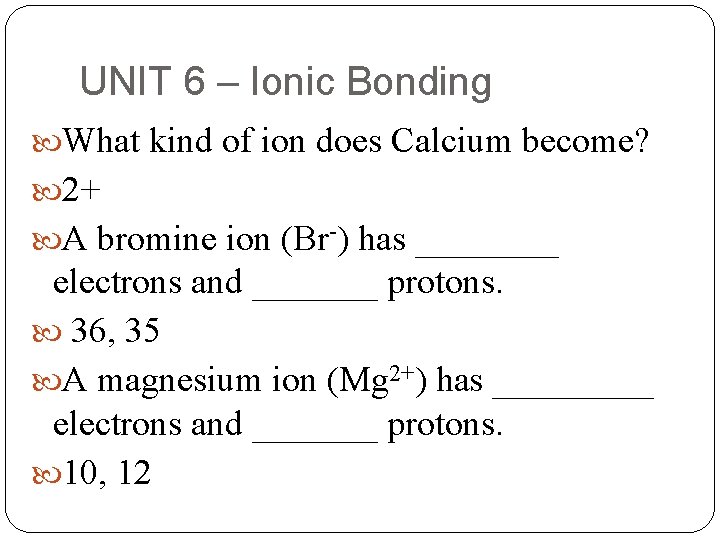
UNIT 6 – Ionic Bonding What kind of ion does Calcium become? 2+ A bromine ion (Br-) has ____ electrons and _______ protons. 36, 35 A magnesium ion (Mg 2+) has _____ electrons and _______ protons. 10, 12

UNIT 6 – Ionic Bonding A cation is a _____ charged ion. Positively An anion is a _____ charged ion. Negatively Ionic bonds form between ___ &_____. Positive & negative ions Metals & Nonmetals

UNIT 6 – Ionic Bonding Ionic compounds have ____ melting points because of the strong forces within. High An alloy is a mixture of two _____. Metals

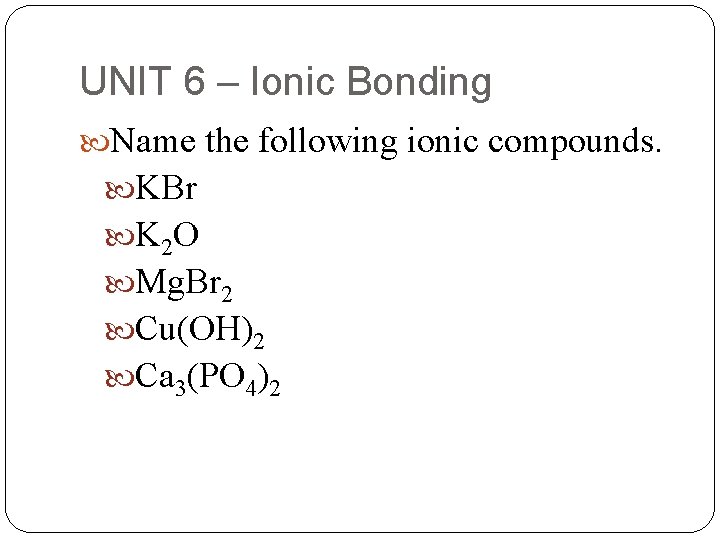
UNIT 6 – Ionic Bonding Name the following ionic compounds. KBr K 2 O Mg. Br 2 Cu(OH)2 Ca 3(PO 4)2

UNIT 6 – Ionic Bonding Write formulas for the following ionic compounds. Copper (I) oxygen Aluminum oxide Lead (IV) sulfide Lead (IV) nitride Lithium chloride

2009 SOL Question How many electrons does the iron ion have when it forms the ionic compound Fe. Cl 3? F 20 G 23. H 26 J 29

UNIT 7 – Covalent Bonding When two nonmetals join together, they form a covalent bond by __ their electrons. Sharing Most atoms that form covalent bonds connect so each atom has _____ valence electrons. 8

UNIT 7 – Covalent Bonding Name the 7 diatomic elements Name the following molecular compounds. N 2 O CO 2 C 2 H 6 H 2 S N 2 O 4

UNIT 7 – Covalent Bonding Write the chemical formula for each of the following molecular compounds. Carbon monoxide Dihydrogen monoxide Phosphorus trichloride Carbon tetrahydride Dicarbon tetrahydride

UNIT 7 – Covalent Bonding Draw the Lewis structure or structural formula of each of the following and determine its shape. Si. Cl 4 SCl 2 PF 3 As. F 5 BF 3

UNIT 7 – Covalent Bonding If there is a LARGE difference in electronegativity, it will result in a ______ bond. Ionic A medium difference in electronegativity will result in a _____ covalent bond. Polar A very small difference will result in a ____ covalent bond. Nonpolar

UNIT 8 – Chemical Reactions The chemicals used to start a chemical reaction are called the _____ Reactants The chemicals that come out of a reaction are the _____ Products If something is written above the arrow, it is a _____ Catalyst

UNIT 8 – Chemical Reactions Balance the following reactions. a. Cu. Cl 2 + H 2 S Cu. S + HCl B. Fe + O 2 Fe 2 O 3 c. P + O 2 P 4 O 10 d. Na + H 2 O H 2 + Na. OH e. Fe. Cl 3 + Ca. OH Fe(OH)3 + Ca. Cl

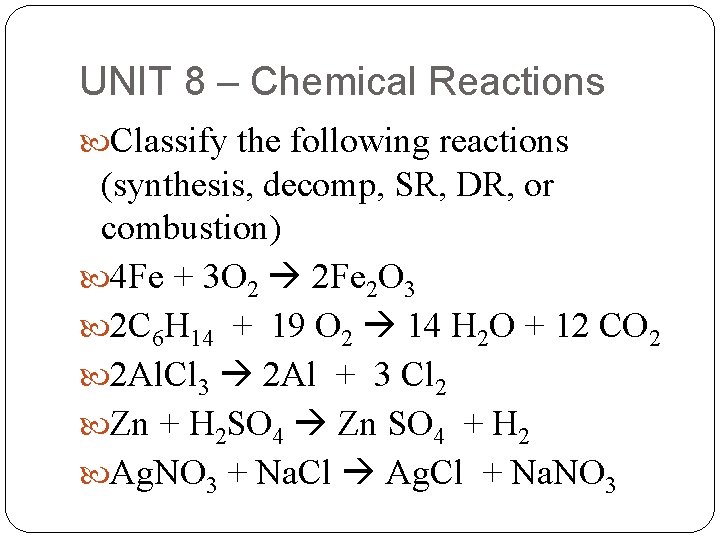
UNIT 8 – Chemical Reactions Classify the following reactions (synthesis, decomp, SR, DR, or combustion) 4 Fe + 3 O 2 2 Fe 2 O 3 2 C 6 H 14 + 19 O 2 14 H 2 O + 12 CO 2 2 Al. Cl 3 2 Al + 3 Cl 2 Zn + H 2 SO 4 Zn SO 4 + H 2 Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3

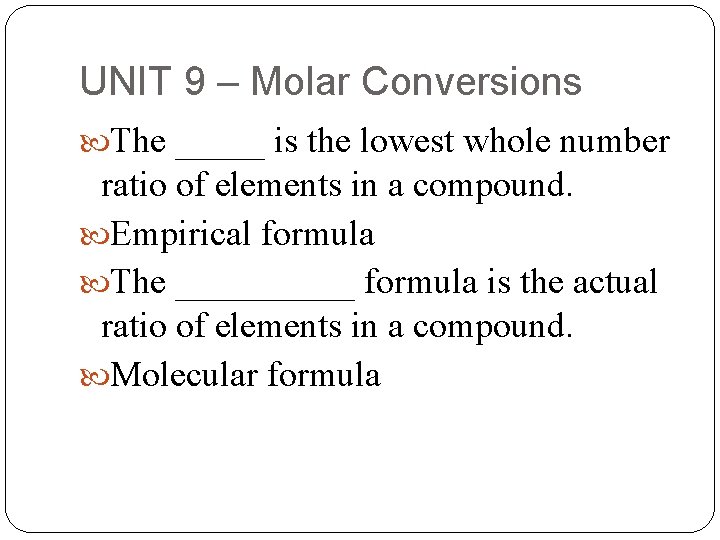
UNIT 9 – Molar Conversions The _____ is the lowest whole number ratio of elements in a compound. Empirical formula The _____ formula is the actual ratio of elements in a compound. Molecular formula

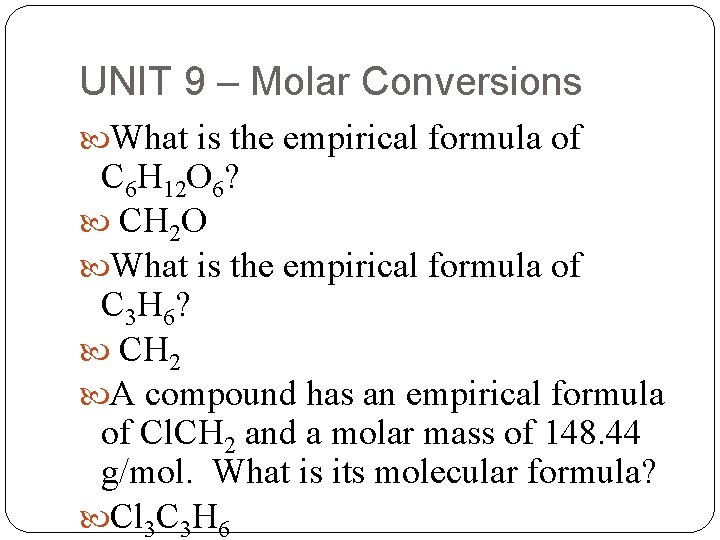
UNIT 9 – Molar Conversions What is the empirical formula of C 6 H 12 O 6? CH 2 O What is the empirical formula of C 3 H 6? CH 2 A compound has an empirical formula of Cl. CH 2 and a molar mass of 148. 44 g/mol. What is its molecular formula? Cl 3 C 3 H 6

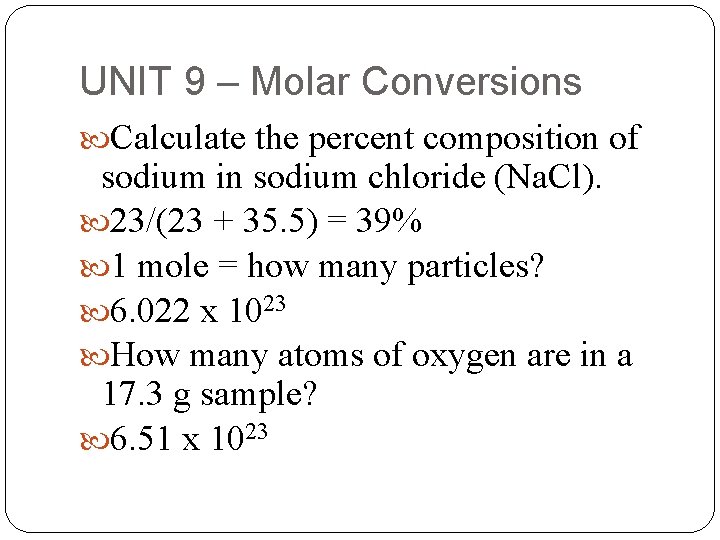
UNIT 9 – Molar Conversions Calculate the percent composition of sodium in sodium chloride (Na. Cl). 23/(23 + 35. 5) = 39% 1 mole = how many particles? 6. 022 x 1023 How many atoms of oxygen are in a 17. 3 g sample? 6. 51 x 1023