chemistry Slide 1 of 39 10 2 MoleMass





















- Slides: 39

chemistry Slide 1 of 39

10. 2 Mole–Mass and Mole– Volume Relationships > The Mole–Mass Relationship How do you convert the mass of a substance to the number of moles of the substance? Slide 2 of 39 © Copyright Pearson Prentice Hall

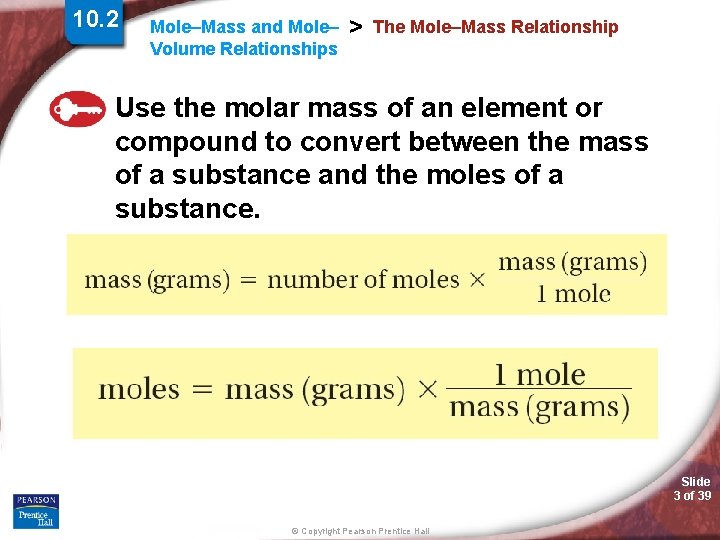
10. 2 Mole–Mass and Mole– Volume Relationships > The Mole–Mass Relationship Use the molar mass of an element or compound to convert between the mass of a substance and the moles of a substance. Slide 3 of 39 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 10. 5 Slide 4 of 39 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 10. 5 Slide 5 of 39 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 10. 5 Slide 6 of 39 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 10. 5 Slide 7 of 39 © Copyright Pearson Prentice Hall

Practice Problems for Sample Problem 10. 5 Problem Solving 10. 16 Solve Problem 16 with the help of an interactive guided tutorial. Slide 8 of 39 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 10. 6 Slide 9 of 39 © Copyright Pearson Prentice Hall

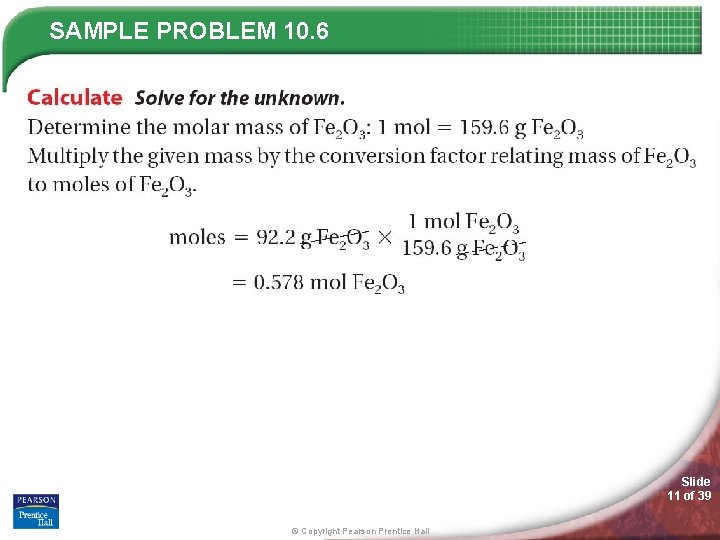
SAMPLE PROBLEM 10. 6 Slide 10 of 39 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 10. 6 Slide 11 of 39 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 10. 6 Slide 12 of 39 © Copyright Pearson Prentice Hall

Practice Problems for Sample Problem 10. 6 Problem Solving 10. 18 Solve Problem 18 with the help of an interactive guided tutorial. Slide 13 of 39 © Copyright Pearson Prentice Hall

10. 2 Mole–Mass and Mole– Volume Relationships > The Mole–Volume Relationship What is the volume of a gas at STP? Slide 14 of 39 © Copyright Pearson Prentice Hall

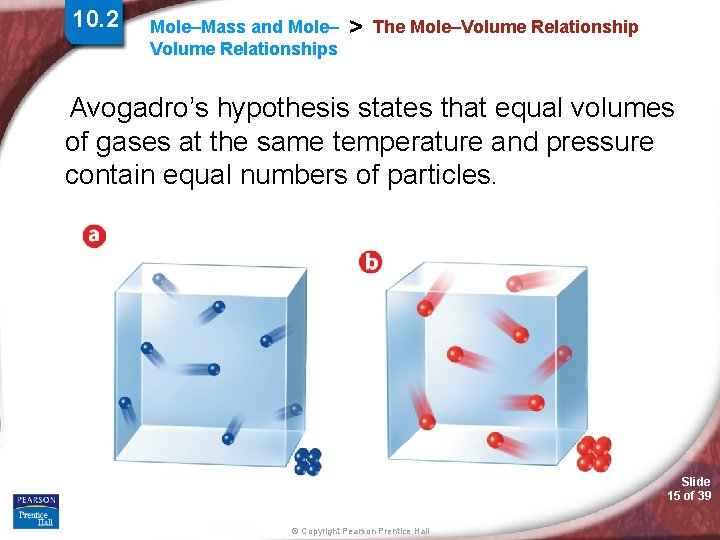
10. 2 Mole–Mass and Mole– Volume Relationships > The Mole–Volume Relationship Avogadro’s hypothesis states that equal volumes of gases at the same temperature and pressure contain equal numbers of particles. Slide 15 of 39 © Copyright Pearson Prentice Hall

10. 2 Mole–Mass and Mole– Volume Relationships > The Mole–Volume Relationship The volume of a gas varies with temperature and pressure. Because of these variations, the volume of a gas is usually measured at a standard temperature and pressure. Standard temperature and pressure (STP) means a temperature of 0°C and a pressure of 101. 3 k. Pa, or 1 atmosphere (atm). Slide 16 of 39 © Copyright Pearson Prentice Hall

10. 2 Mole–Mass and Mole– Volume Relationships > The Mole–Volume Relationship At STP, 1 mol or, 6. 02 1023 representative particles, of any gas occupies a volume of 22. 4 L. The quantity 22. 4 L is called the molar volume of a gas. Slide 17 of 39 © Copyright Pearson Prentice Hall

10. 2 Mole–Mass and Mole– Volume Relationships > The Mole–Volume Relationship Calculating Volume at STP Slide 18 of 39 © Copyright Pearson Prentice Hall

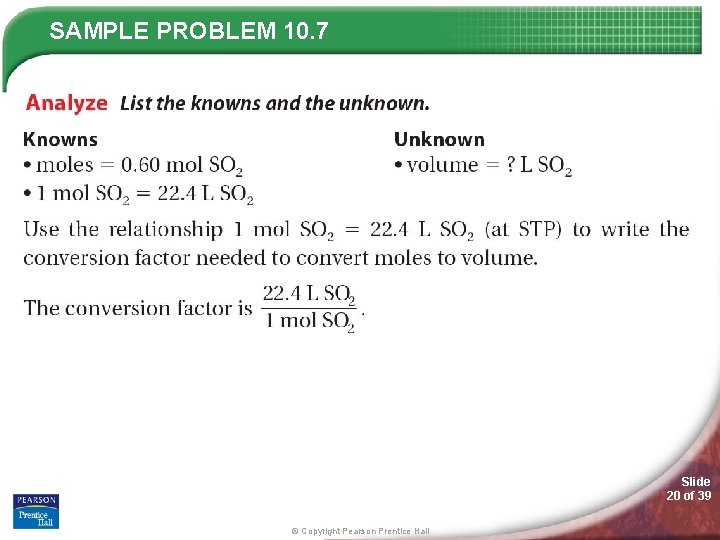
SAMPLE PROBLEM 10. 7 Slide 19 of 39 © Copyright Pearson Prentice Hall

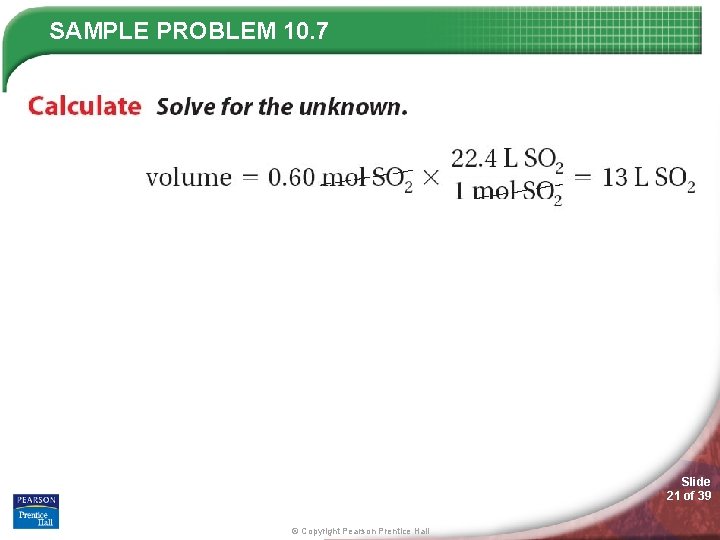
SAMPLE PROBLEM 10. 7 Slide 20 of 39 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 10. 7 Slide 21 of 39 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 10. 7 Slide 22 of 39 © Copyright Pearson Prentice Hall

Practice Problems for Sample Problem 10. 7 Problem Solving 10. 20 Solve Problem 20 with the help of an interactive guided tutorial. Slide 23 of 39 © Copyright Pearson Prentice Hall

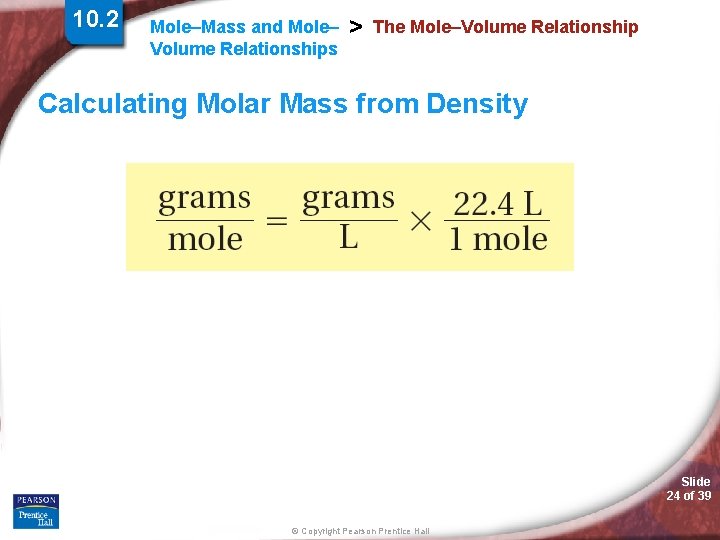
10. 2 Mole–Mass and Mole– Volume Relationships > The Mole–Volume Relationship Calculating Molar Mass from Density Slide 24 of 39 © Copyright Pearson Prentice Hall

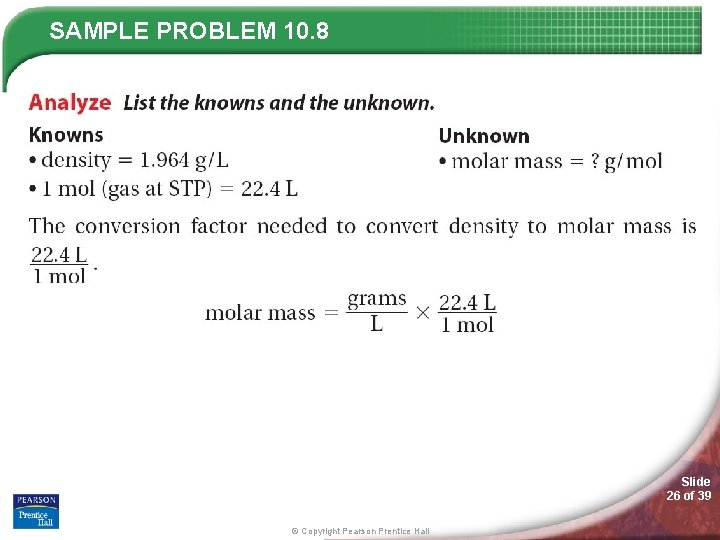
SAMPLE PROBLEM 10. 8 Hint: Molar mass means you are looking for grams/mole! Slide 25 of 39 © Copyright Pearson Prentice Hall

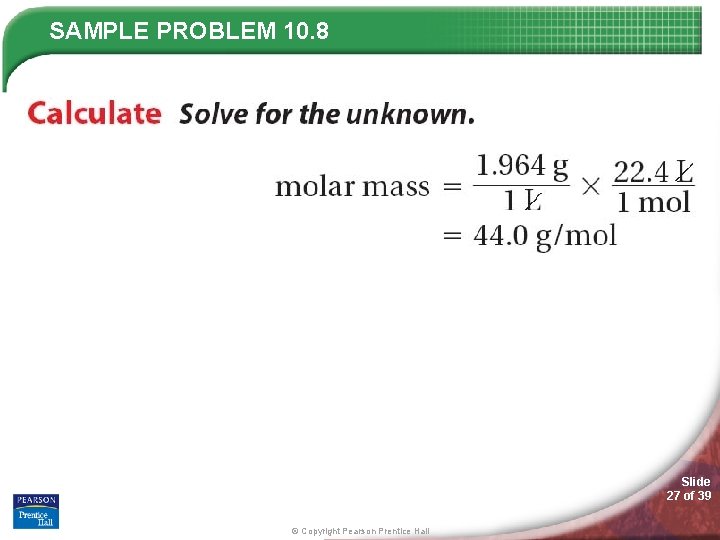
SAMPLE PROBLEM 10. 8 Slide 26 of 39 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 10. 8 Slide 27 of 39 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 10. 8 Slide 28 of 39 © Copyright Pearson Prentice Hall

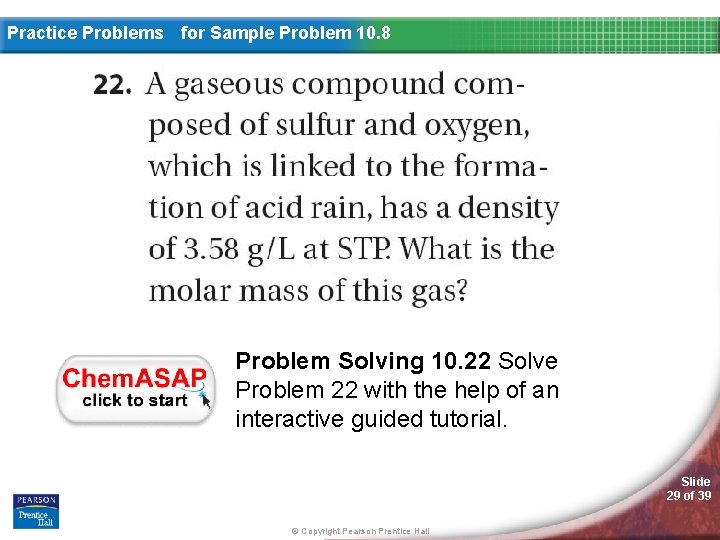
Practice Problems for Sample Problem 10. 8 Problem Solving 10. 22 Solve Problem 22 with the help of an interactive guided tutorial. Slide 29 of 39 © Copyright Pearson Prentice Hall

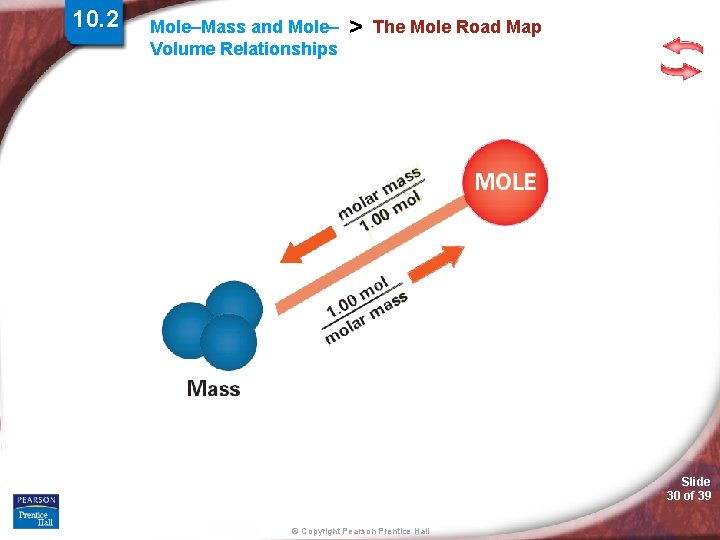
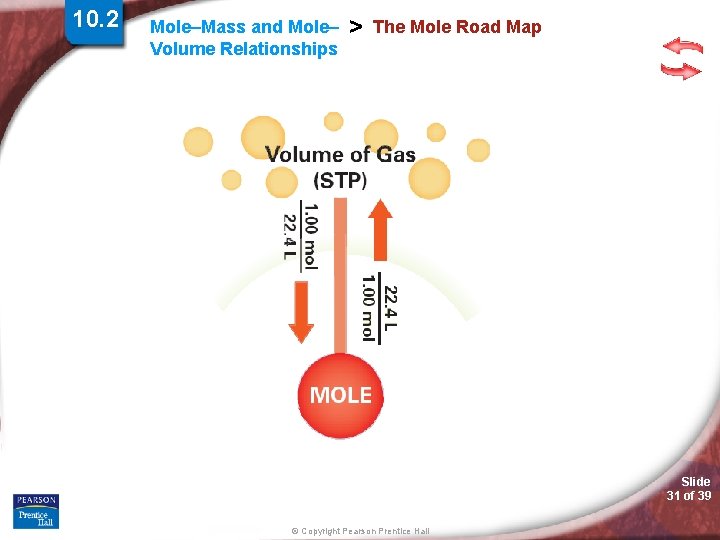
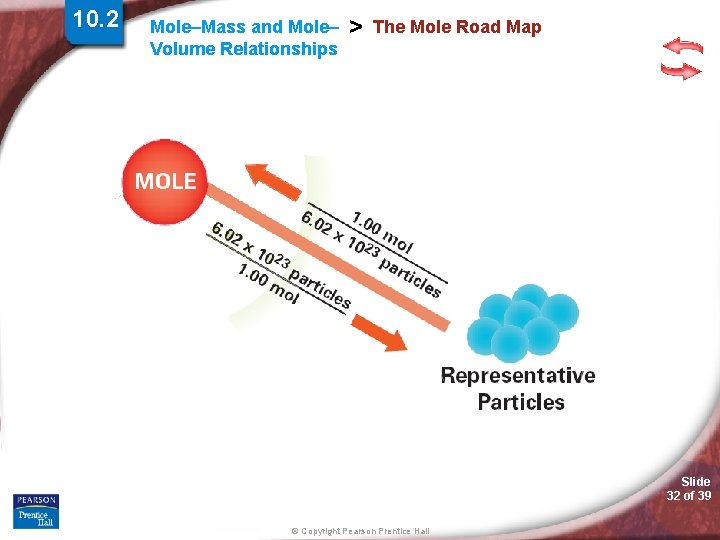
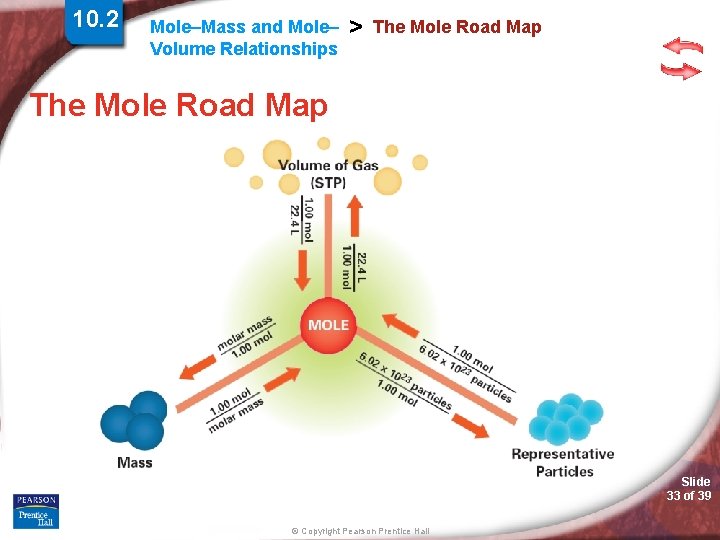
10. 2 Mole–Mass and Mole– Volume Relationships > The Mole Road Map Slide 30 of 39 © Copyright Pearson Prentice Hall

10. 2 Mole–Mass and Mole– Volume Relationships > The Mole Road Map Slide 31 of 39 © Copyright Pearson Prentice Hall

10. 2 Mole–Mass and Mole– Volume Relationships > The Mole Road Map Slide 32 of 39 © Copyright Pearson Prentice Hall

10. 2 Mole–Mass and Mole– Volume Relationships > The Mole Road Map Slide 33 of 39 © Copyright Pearson Prentice Hall

Mole–Mass and Mole– Volume Relationships > Simulation 10 Use the mole road map to convert among mass, volume, and number of representative particles. Slide 34 of 39 © Copyright Pearson Prentice Hall

10. 2 Section Quiz. Assess students’ understanding of the concepts in Section 10. 2. Continue to: -or- Launch: Section Quiz Slide 35 of 39 © Copyright Pearson Prentice Hall

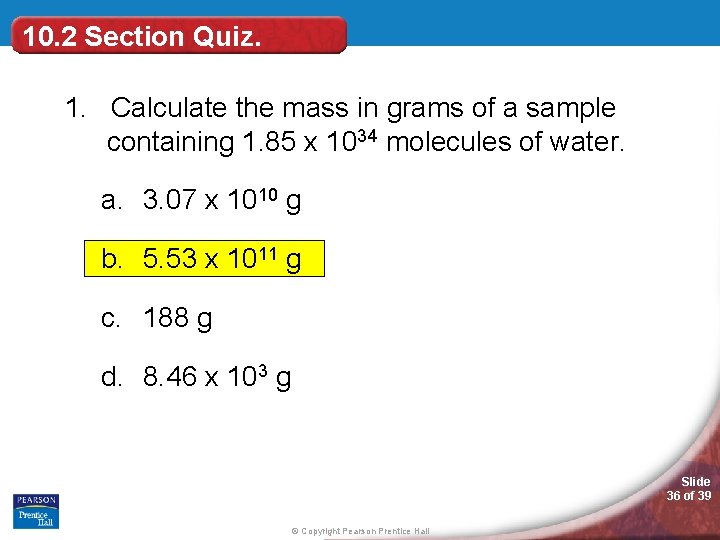
10. 2 Section Quiz. 1. Calculate the mass in grams of a sample containing 1. 85 x 1034 molecules of water. a. 3. 07 x 1010 g b. 5. 53 x 1011 g c. 188 g d. 8. 46 x 103 g Slide 36 of 39 © Copyright Pearson Prentice Hall

10. 2 Section Quiz. 2. Calculate the number of moles in a spoonful of table sugar (C 12 H 22 O 11) having a mass of 10. 5 g. a. 32. 6 mol b. 3. 59 103 mol c. 3. 07 10– 3 mol d. 1. 85 1022 mol Slide 37 of 39 © Copyright Pearson Prentice Hall

10. 2 Section Quiz. 3. What is the volume of 0. 35 mol of oxygen gas at STP? a. 32 L b. 64 L c. 7. 8 L d. 16 L Slide 38 of 39 © Copyright Pearson Prentice Hall

END OF SHOW
 Molemass
Molemass What are the basic dance steps in heel and toe polka
What are the basic dance steps in heel and toe polka How to facot
How to facot Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Slide to doc.com
Slide to doc.com Mississippi cha cha slide
Mississippi cha cha slide Housekeeping slide for webinar
Housekeeping slide for webinar Slidetodoc.com
Slidetodoc.com Vroom slide system
Vroom slide system Mola hidatiforme fotos
Mola hidatiforme fotos Auditoria em enfermagem slide
Auditoria em enfermagem slide Norma abnt slide
Norma abnt slide Mausculas
Mausculas Four types of transformations
Four types of transformations Bisantrene
Bisantrene Source documents images
Source documents images Slide todoc.com
Slide todoc.com Slide todoc.com
Slide todoc.com Latin ng gratitude
Latin ng gratitude Slide ambrogio
Slide ambrogio Presentation outline slide
Presentation outline slide Embryo slide culture dish
Embryo slide culture dish Prova orale tfa sostegno domanda motivazionale
Prova orale tfa sostegno domanda motivazionaleSlidetodoc downloader
 Disclosure slides
Disclosure slides Disclosure slides
Disclosure slides Slip slide collide game
Slip slide collide game What are rights
What are rights Present simple slide
Present simple slide Slide is an example of simple machine
Slide is an example of simple machine Slide to doc.com
Slide to doc.com Slide selamat datang
Slide selamat datang Alide mustafayeva
Alide mustafayeva Disclosure slide example
Disclosure slide example Slide sal da terra e luz do mundo
Slide sal da terra e luz do mundo Advantages of robots
Advantages of robots Slidetodoc.com
Slidetodoc.com Slide to doc.com
Slide to doc.com Slidetodoc
Slidetodoc