Chemistry Skills Check 1 1 SKILLS CHECK ON












- Slides: 22

Chemistry- Skills Check 1 1

SKILLS CHECK ON THE FOLLOWING MIXTURES Homogeneous Heterogeneous Solutions Suspensions Colloids Emulsions

Homogeneous Mixture ? #1 Which of the following is a homogeneous solution? A. B. C. D. 3

Heterogeneous Mixture ? #2 Which of the following is a heterogeneous mixture? A. B. C. D. 4

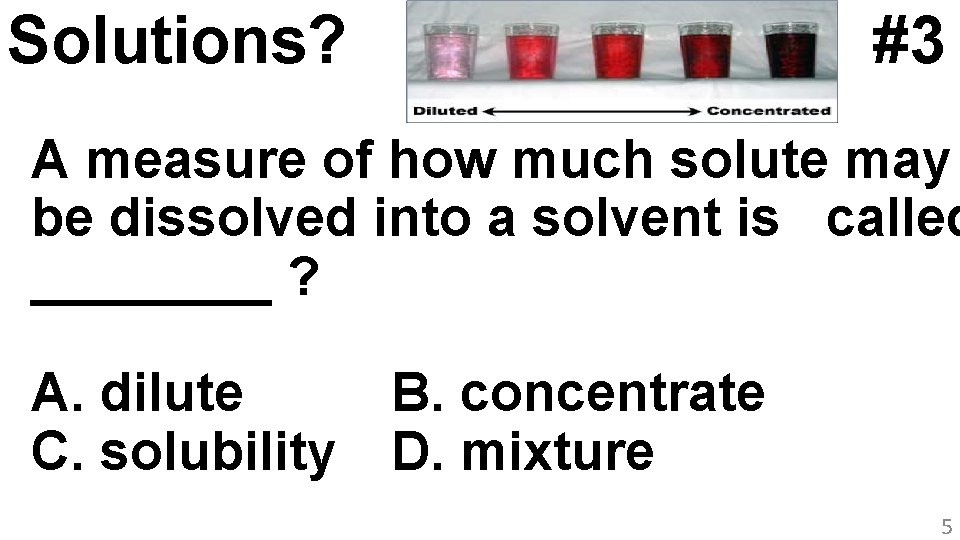
Solutions? #3 A measure of how much solute may be dissolved into a solvent is called ____ ? A. dilute C. solubility B. concentrate D. mixture 5

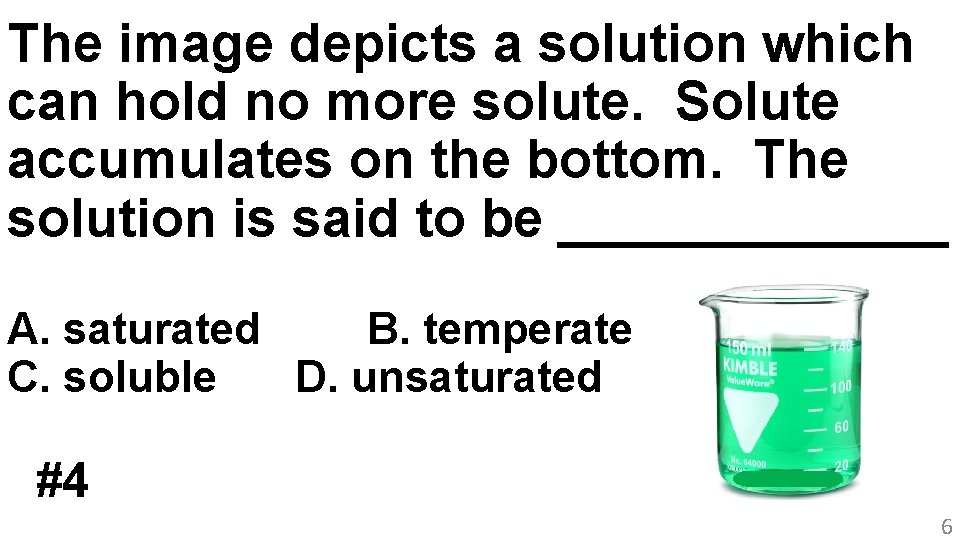
The image depicts a solution which can hold no more solute. Solute accumulates on the bottom. The solution is said to be _______ A. saturated B. temperate C. soluble D. unsaturated #4 6

Suspensions? #5 Suspensions differ from solutions in several ways. List all that apply. a. Clear or b. cloudy c. Particles settle or d. don’t settle e. Particles visible or f. not visible g. Water vapor or h. dust – example i. Is a mixture or j. is not a mixture 7

Solutions? #6 All of the following are ways to increase solubility of a solute except. a. Increase heat & increase solvent b. filtration & evaporation c. crush or grind to increase surface area d. stirring & increase heat 8

Suspensions? #7 All of the following are ways to separate suspensions discussed in your text and demonstrated in class, except? Settling, Boiling, Filtration, Coagulation Separation by Centrifuge, Evaporation 9

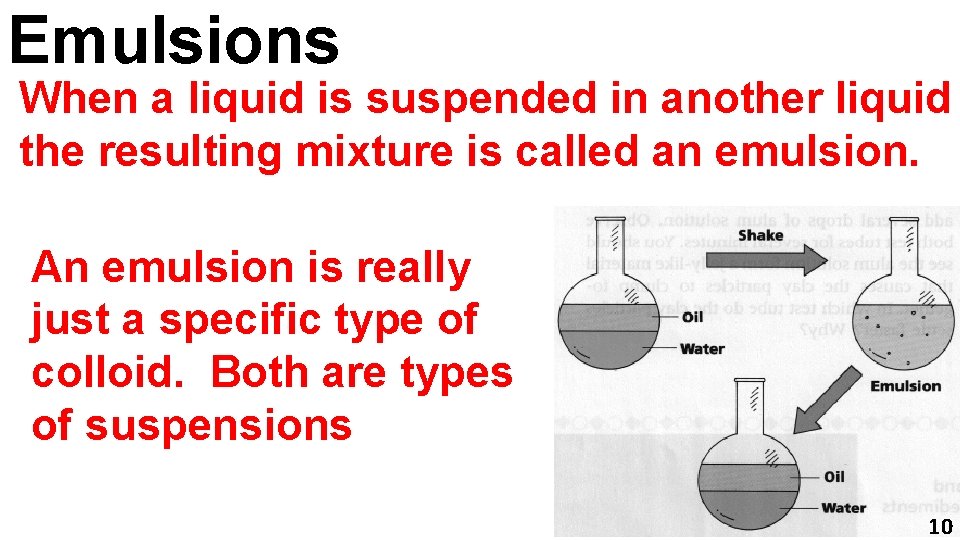
Emulsions When a liquid is suspended in another liquid the resulting mixture is called an emulsion. An emulsion is really just a specific type of colloid. Both are types of suspensions 10

Colloids These are suspensions in which the particles are permanently suspended. Colloids do not separate when left standing. Some permanent emulsions are colloids. The particles in a colloid are larger than those of solution, however smaller than those of suspensions. Another way to think of a colloid is a suspension that cannot be separated by filtration. 11

Example A. Solution B. Solute #8 C. Suspension D. Solvent Homogeneous or Heterogeneous 12

Example A. Solution B. Colloid #9 C. Suspension D. Emulsion Homogeneous or Heterogeneous 13

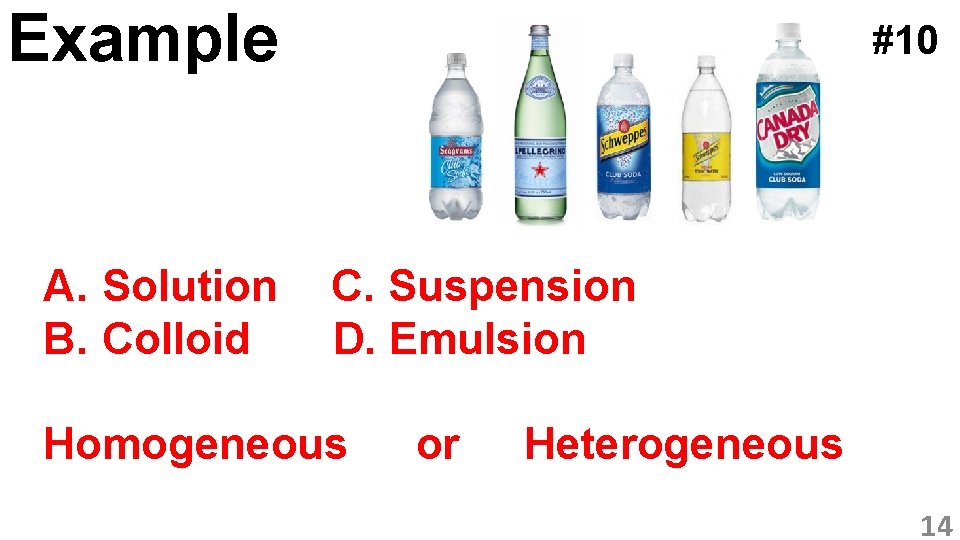
Example A. Solution B. Colloid #10 C. Suspension D. Emulsion Homogeneous or Heterogeneous 14

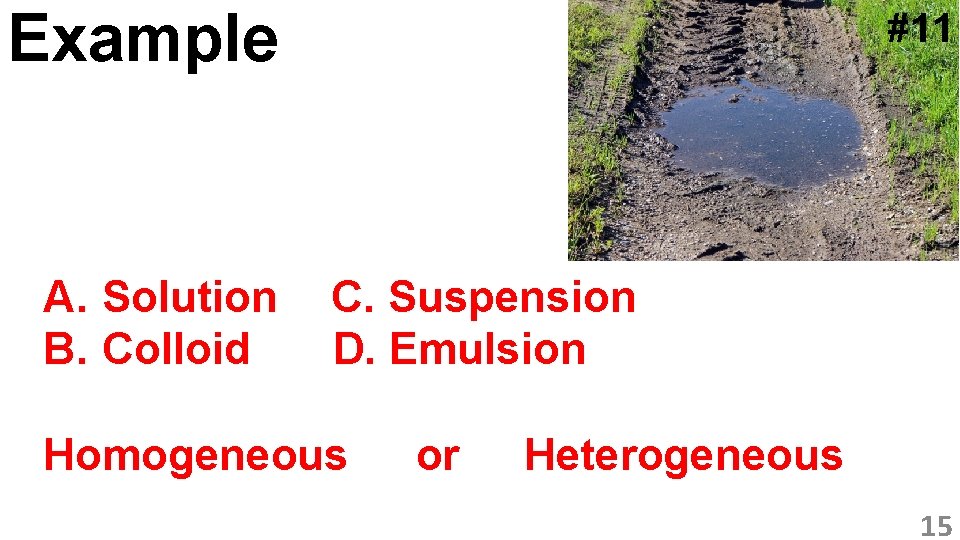
Example A. Solution B. Colloid #11 C. Suspension D. Emulsion Homogeneous or Heterogeneous 15

Example – homogenized milk A. Solution B. Colloid #12 C. Solvent D. Solute Homogeneous or Heterogeneous Bonus: hint – temp or permanent this… 16

#13 Example – fresh milk A. Solvent B. Colloid C. Solute D. Temporary Emulsion Homogeneous or Heterogeneous 17

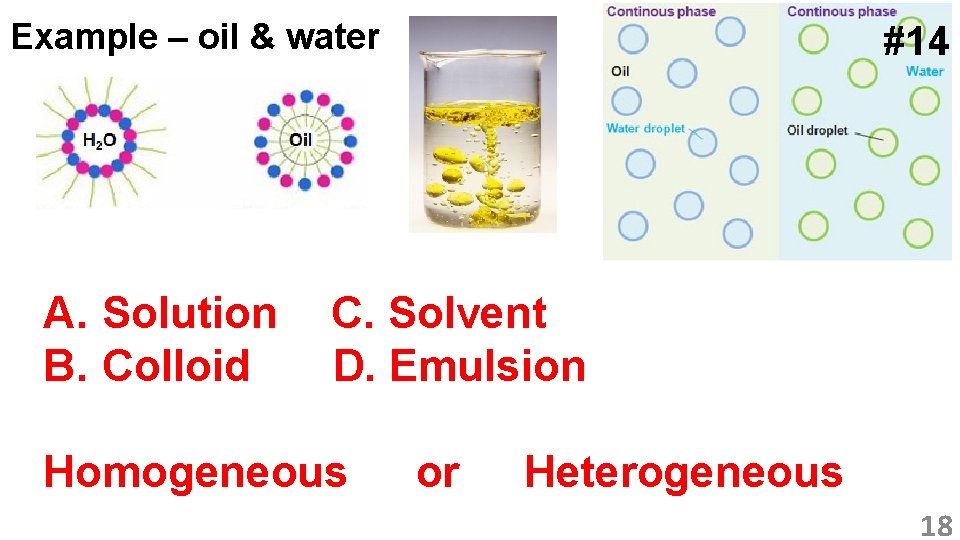
Example – oil & water A. Solution B. Colloid #14 C. Solvent D. Emulsion Homogeneous or Heterogeneous 18

Example – oil & vinegar A. Solution B. Colloid #15 C. Solvent D. Emulsion Homogeneous or Heterogeneous 19

Example A. Solvent B. Colloid #16 C. Suspension D. Emulsion Homogeneous or Heterogeneous 20

Example - fog A. Solution B. Colloid #17 C. Solvent D. Emulsion Homogeneous or Heterogeneous 21

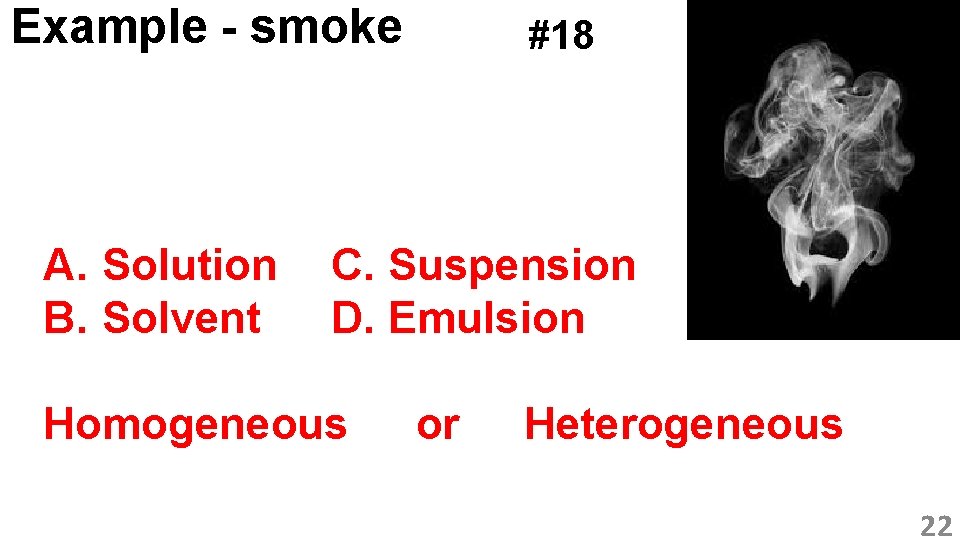
Example - smoke A. Solution B. Solvent #18 C. Suspension D. Emulsion Homogeneous or Heterogeneous 22
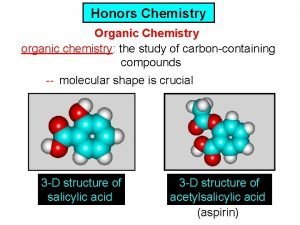
 Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Behavior check in check out sheet
Behavior check in check out sheet Behavior check in check out sheet
Behavior check in check out sheet Check in check out
Check in check out Check in check out system for students
Check in check out system for students Jobbank
Jobbank Dda line drawing algorithm
Dda line drawing algorithm Check in check out pbis
Check in check out pbis Check in and check out intervention
Check in and check out intervention Quickchek menu
Quickchek menu How to endorse a check to someone else
How to endorse a check to someone else Check your progress 1
Check your progress 1 Skills check mathsbox answers
Skills check mathsbox answers Intrapersonal skills definition
Intrapersonal skills definition Soft skills это
Soft skills это Skills passport
Skills passport How to calculate percentage yield in organic chemistry
How to calculate percentage yield in organic chemistry You light up my life chemistry lab answer key
You light up my life chemistry lab answer key Theoretical yueld
Theoretical yueld Naming ionic compounds criss cross method
Naming ionic compounds criss cross method Chemistry abstract example
Chemistry abstract example Abstract and keywords example
Abstract and keywords example