Chemistry Review 1 Elements A 90 occur naturally


- Slides: 13

Chemistry Review

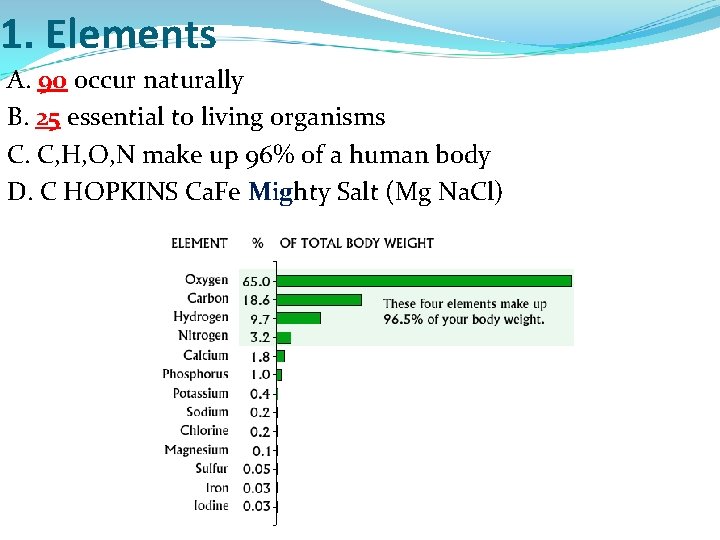
1. Elements A. 90 occur naturally B. 25 essential to living organisms C. C, H, O, N make up 96% of a human body D. C HOPKINS Ca. Fe Mighty Salt (Mg Na. Cl)

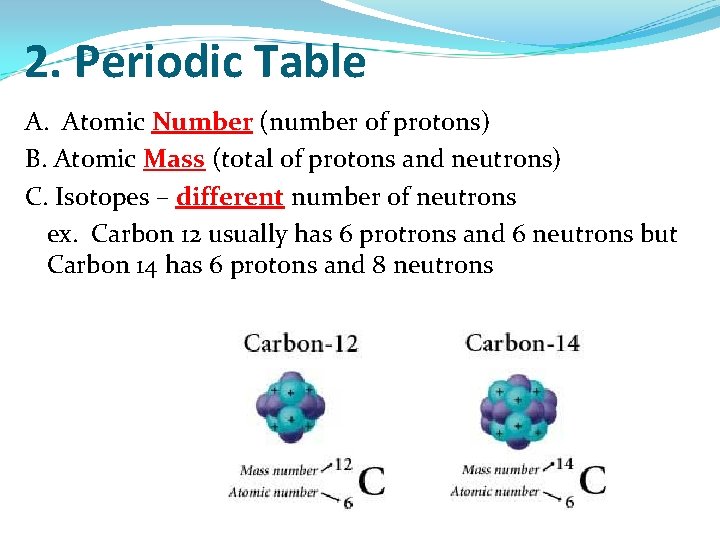
2. Periodic Table A. Atomic Number (number of protons) B. Atomic Mass (total of protons and neutrons) C. Isotopes – different number of neutrons ex. Carbon 12 usually has 6 protrons and 6 neutrons but Carbon 14 has 6 protons and 8 neutrons


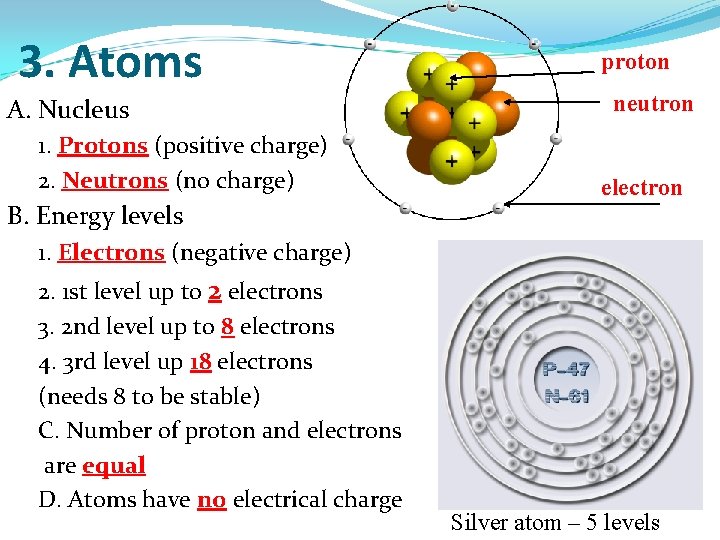
3. Atoms A. Nucleus 1. Protons (positive charge) 2. Neutrons (no charge) B. Energy levels proton neutron electron 1. Electrons (negative charge) 2. 1 st level up to 2 electrons 3. 2 nd level up to 8 electrons 4. 3 rd level up 18 electrons (needs 8 to be stable) C. Number of proton and electrons are equal D. Atoms have no electrical charge Silver atom – 5 levels

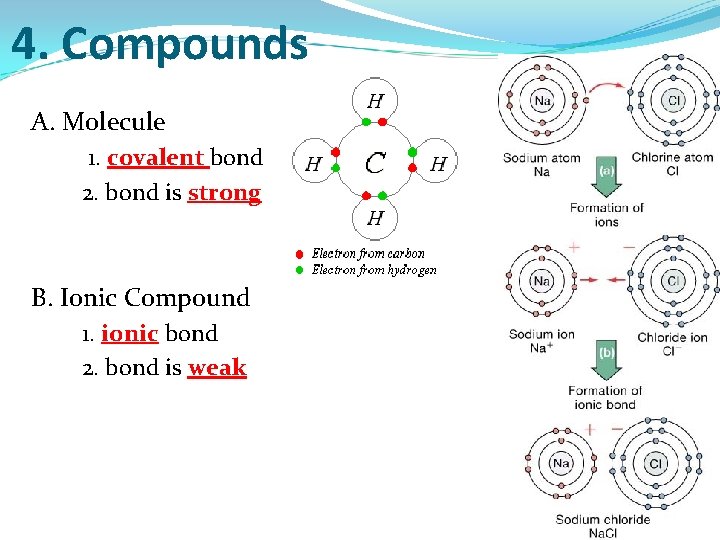
4. Compounds A. Molecule 1. covalent bond 2. bond is strong B. Ionic Compound 1. ionic bond 2. bond is weak

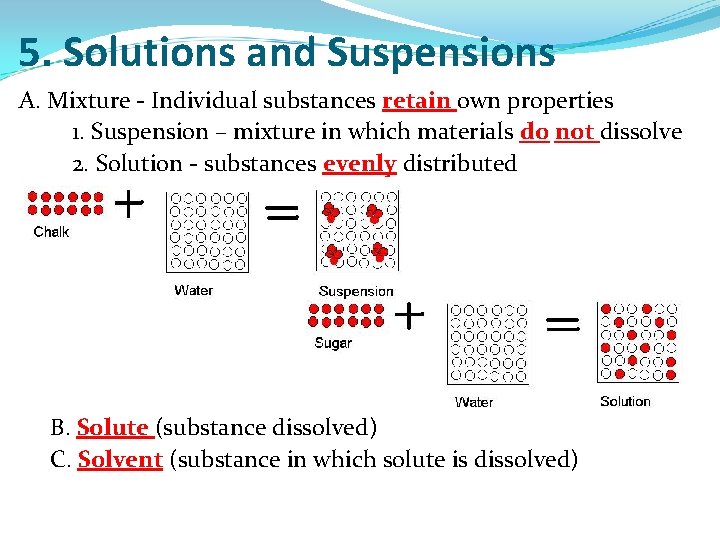
5. Solutions and Suspensions A. Mixture - Individual substances retain own properties 1. Suspension – mixture in which materials do not dissolve 2. Solution - substances evenly distributed B. Solute (substance dissolved) C. Solvent (substance in which solute is dissolved)

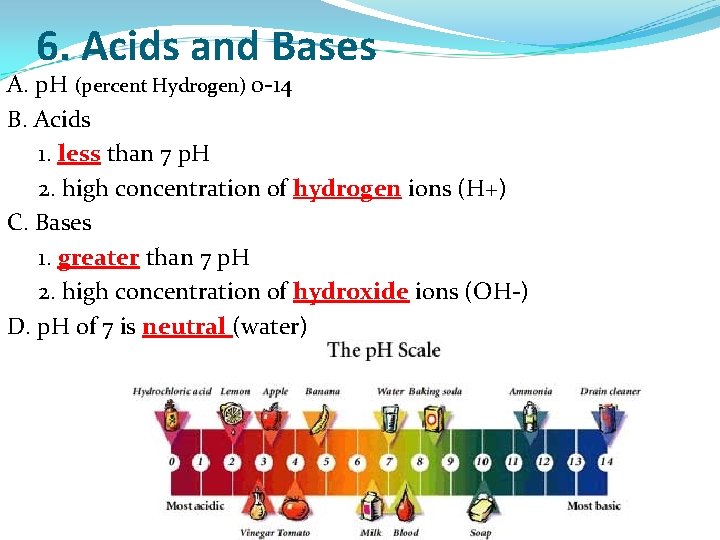
6. Acids and Bases A. p. H (percent Hydrogen) 0 -14 B. Acids 1. less than 7 p. H 2. high concentration of hydrogen ions (H+) C. Bases 1. greater than 7 p. H 2. high concentration of hydroxide ions (OH-) D. p. H of 7 is neutral (water)

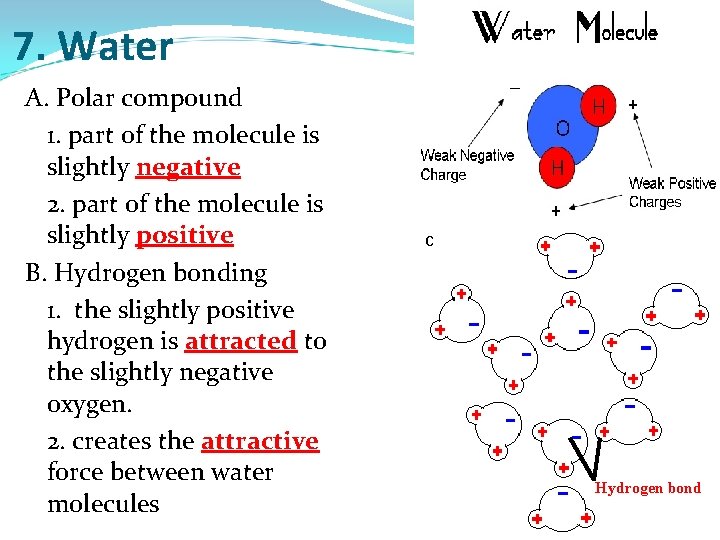
7. Water A. Polar compound 1. part of the molecule is slightly negative 2. part of the molecule is slightly positive B. Hydrogen bonding 1. the slightly positive hydrogen is attracted to the slightly negative oxygen. 2. creates the attractive force between water molecules Hydrogen bond

C. Cohesion - Water is attracted to other water molecules D. Adhesion - Water can also be attracted to other materials. E. Capillary action - the ability of a substance to draw another substance into it. Adhesive forces are stronger than cohesive forces. Plants rely upon capillary action to draw water from soil into roots.

F. Universal solvent 1. carries nutrients 2. removes wastes G. Most abundant substance in body 1. Needed for digestion 2. Regulates temperature

8. Chemical Reactions A. Reactants - materials that will react with one another B. Products - materials produced in the reaction C. Catalyst – substance, usually used in small amounts relative to the reactants, that modifies and increases the rate of a reaction without being consumed in the process D. Metabolism – the set of chemical reactions that occur in living organisms in order to maintain life. C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + energy (Respiration) C 6 H 12 O 6 + 6 O 2 (Photosynthesis)

The End