Chemistry project To determine the percentage of calcium

























- Slides: 34

Chemistry project • To determine the percentage of calcium carbonate in eggshell • • • Lin Yin Shing (23) Hung Ching Yin (4) Wai Sze Wah (8) Poon Yee Man (7) Yuen Chun Fai (30)

Content • • • Introduction Principle of this experiment Apparatus and equipments Experiment procedures Chemical used Sample used Source of errors Assumption made Experimental results Calculations Conclusion Sources of references

Introduction • 1960's - 1970's USA used a pesticide, DDT extensively • Run-off from DDT entered our waterways and eventually into many of our wild birdlife. • DDT affected the population by weakening the eggshells which would break before hatching. • An example of this devastation was the American Bald Eagle whose population was as low as 400 mating pairs in the lower 48 states. • DDT has been banned in the USA and the Bald Eagle is no longer on the endangered species list. One method of monitoring the strength of the egg is by determining the percentage of calcium carbonate in the eggshell. • This can be accomplished through an acid/base titration method.

Principle of the experiment • During this lab, the percentage of Ca. CO 3 in an eggshell is determined by reacting the eggshell with hydrochloric acid. The equation for this reaction is: • 2 HCl (aq) + Ca. CO 3(s) →Ca 2+ (aq) + CO 2 (g) + H 2 O (l) + 2 Cl- (aq) • • This reaction cannot be used directly titrate with the Ca. CO 3. Instead, an excess of hydrochloric acid is added to dissolve the eggshell, and the remaining acid is titrated with Na. OH solution to determine the amount of acid that did not react with the eggshell. The equation used to determine the amount of leftover acid is: • HCl (aq) + Na. OH (aq) → H 2 O (l) + Na+ (aq) + Cl- (aq) •

• In order to help the hydrochloric acid dissolve the Ca. CO 3, ethyl alcohol is added to the eggshell as a wetting agent. Wetting agents are chemicals that increase the spreading and penetrating properties of a liquid by lowering its surface tension—that is, the tendency of its molecules to adhere to each other. • Although it is now banned in the United States, the pesticide DDT has caused significant damage to the environment and its wildlife. Birds are especially affected because the DDT weakens the shells of their eggs, which would break before hatching. This caused certain bird species to become endangered (i. e. the American bald eagle). One method of monitoring the strength of the egg is by determining the percentage of calcium carbonate in the eggshell. • The percentage of calcium carbonate indicate that the strength of the eggshell. And the strength of the eggshell show serious the pesticide damaged to the eggshell.

Apparatus and equipments • 250. 00 cm 3 beaker X 4 • glass rod X 1 • Filter funnel X 1 • Wash bottle X 1 • Electronic balance X 1 • Weighing bottle X 1 • 250. 00 cm 3 volumetric flask X 2 • 25. 00 cm 3 pipette X 2

• Conical flasks X 2 • Measuring cylinder X 1 • Bunsen burner X 2 • Heatproof mat X 1 • Burette X 2 • Wire gaze X 2 • Tripod X 2 • White tile X 2 • Mortar and pestle X 2 • Oven X 1

Chemical used • • 0. 1 M Na. OH 0. 2 M HCl Phenolphthalein Ethanol

Sample used ü 2 different eggs (1 white & 1 brown) USA Malaysia

Procedures 1) The eggs was boiled and cooled down. 2) The protein membrane was removed on the inside of the boiled eggshell. 3) The eggshell washed with distilled water

5) The eggshell was then grounded into fine powder by mortar and pestle. (white egg) (brown egg)

6) Dried in an oven for ten minutes.

7) 0. 2 g of eggshell powder is weighted accurately by using an electronic balance and it was transferred to a conical flask.

8) 25. 00 cm 3 of HCl was pipetted to dissolve the eggshell powder and 5 cm 3 of ethanol was also added in order to help the HCl dissolve the Ca. CO 3 9) Distilled water was added to the flask until it reached about 50 cm 3. The flask was swirled gently.

10) The mixture was boiled for about 15 minutes (boiling away CO 2) and then cooled down.

10) Boil !! 15 minutes!!! Cool down!!

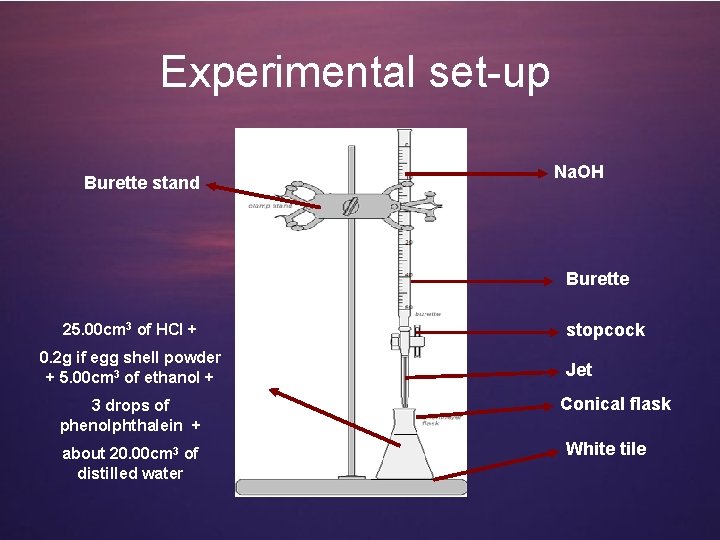
Experimental set-up Burette stand Na. OH Burette 25. 00 cm 3 of HCl + 0. 2 g if egg shell powder + 5. 00 cm 3 of ethanol + 3 drops of phenolphthalein + about 20. 00 cm 3 of distilled water stopcock Jet Conical flask White tile

11) The solution was then titrated with standardized Na. OH. 12) Repeat another sample

Experimental results The colour change of the solution: White egg: Brown egg: Colourless pink

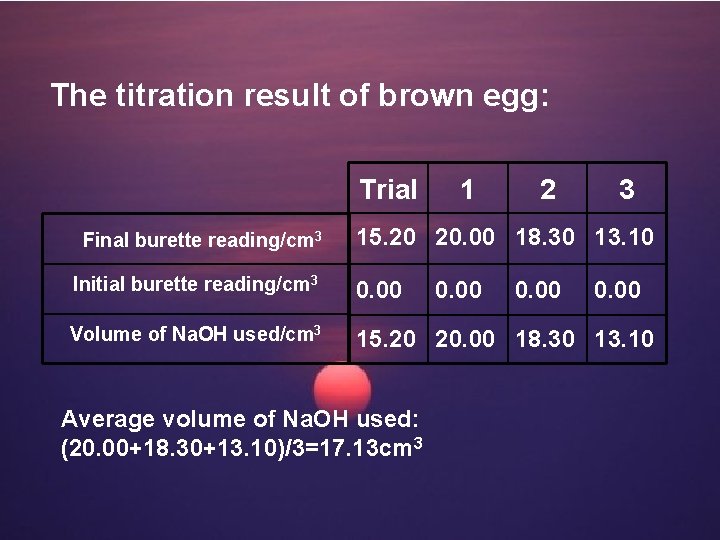
The titration result of brown egg: Trial 1 2 3 Final burette reading/cm 3 15. 20 20. 00 18. 30 13. 10 Initial burette reading/cm 3 0. 00 Volume of Na. OH used/cm 3 15. 20 20. 00 18. 30 13. 10 Average volume of Na. OH used: (20. 00+18. 30+13. 10)/3=17. 13 cm 3 0. 00

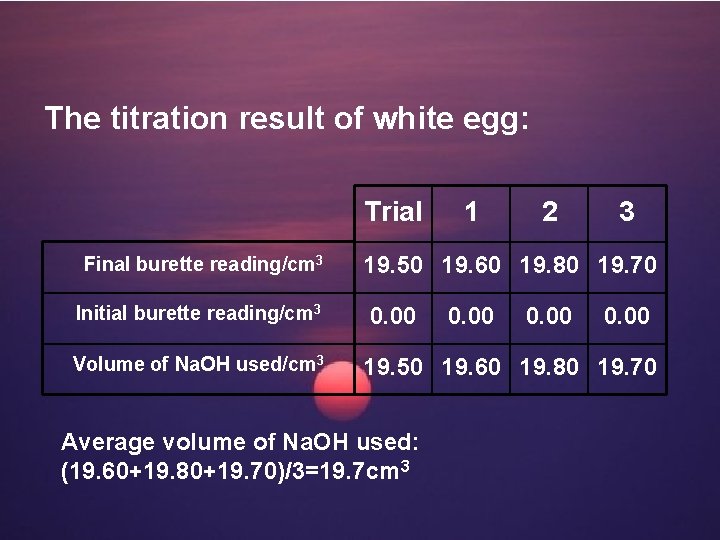
The titration result of white egg: Trial Final burette reading/cm 3 Initial burette reading/cm 3 Volume of Na. OH used/cm 3 1 2 3 19. 50 19. 60 19. 80 19. 70 0. 00 19. 50 19. 60 19. 80 19. 70 Average volume of Na. OH used: (19. 60+19. 80+19. 70)/3=19. 7 cm 3

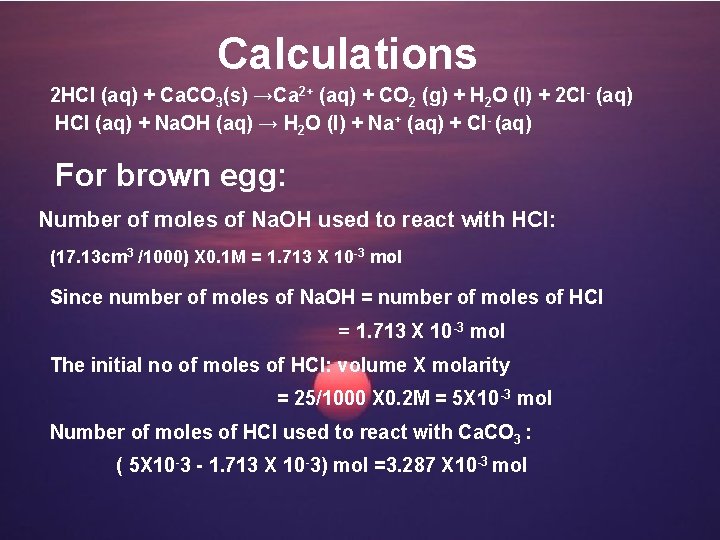
Calculations 2 HCl (aq) + Ca. CO 3(s) →Ca 2+ (aq) + CO 2 (g) + H 2 O (l) + 2 Cl- (aq) HCl (aq) + Na. OH (aq) → H 2 O (l) + Na+ (aq) + Cl- (aq) For brown egg: Number of moles of Na. OH used to react with HCl: (17. 13 cm 3 /1000) X 0. 1 M = 1. 713 X 10 -3 mol Since number of moles of Na. OH = number of moles of HCl = 1. 713 X 10 -3 mol The initial no of moles of HCl: volume X molarity = 25/1000 X 0. 2 M = 5 X 10 -3 mol Number of moles of HCl used to react with Ca. CO 3 : ( 5 X 10 -3 - 1. 713 X 10 -3) mol =3. 287 X 10 -3 mol

According to the equation, one mole of HCI required to react with 2 moles of Ca. CO 3, Number of moles of Ca. CO 3 reacted: 3. 287 X 10 - 3 /2 = 1. 6435 X 10 -3 mol The weight of Ca. CO 3: 1. 6435 X 10 -3 g X 100. 1 =0. 1645 g The % of Ca. CO 3 in eggshell (brown) : (0. 1645/0. 2) X 100% = 82. 25%

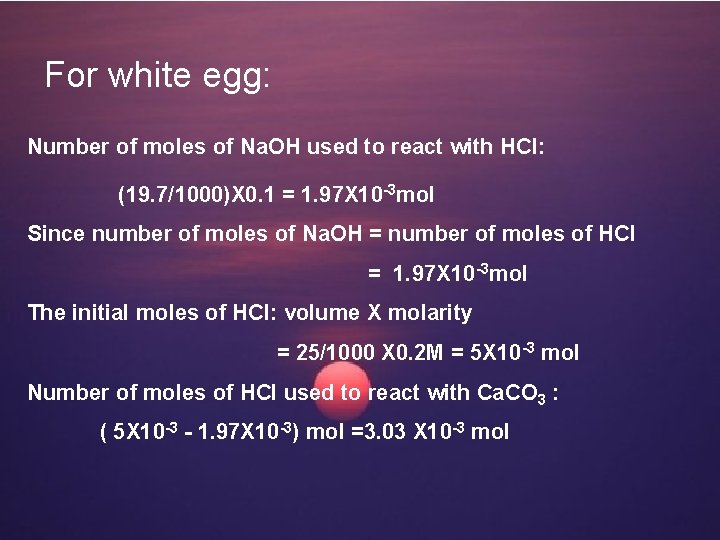
For white egg: Number of moles of Na. OH used to react with HCl: (19. 7/1000)X 0. 1 = 1. 97 X 10 -3 mol Since number of moles of Na. OH = number of moles of HCl = 1. 97 X 10 -3 mol The initial moles of HCl: volume X molarity = 25/1000 X 0. 2 M = 5 X 10 -3 mol Number of moles of HCl used to react with Ca. CO 3 : ( 5 X 10 -3 - 1. 97 X 10 -3) mol =3. 03 X 10 -3 mol

Number of moles of Ca. CO 3 reacted: 3. 03 X 10 -3/2 = 1. 515 X 10 -3 mol The weight of Ca. CO 3: 1. 515 X 10 -3 g X 100. 1 =0. 15165 g The % of Ca. CO 3 in eggshell (white) : (0. 15165/0. 2) X 100% = 78. 83%

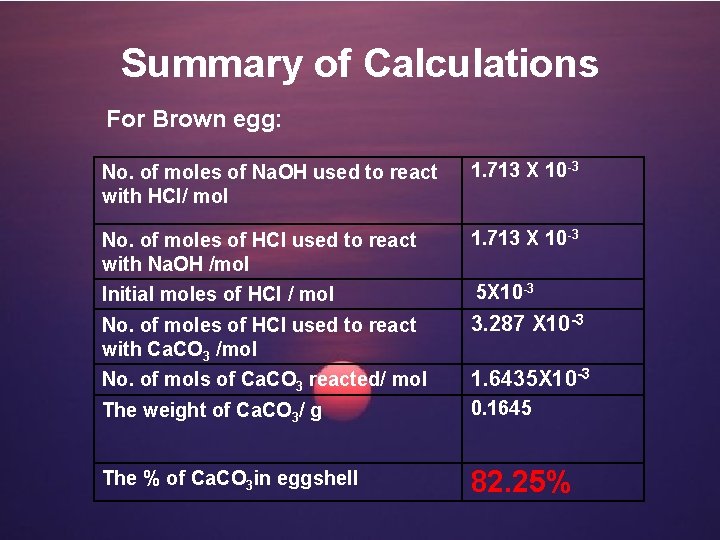
Summary of Calculations For Brown egg: No. of moles of Na. OH used to react with HCl/ mol 1. 713 X 10 -3 No. of moles of HCl used to react with Na. OH /mol 1. 713 X 10 -3 Initial moles of HCl / mol 5 X 10 -3 No. of moles of HCl used to react with Ca. CO 3 /mol 3. 287 X 10 -3 No. of mols of Ca. CO 3 reacted/ mol 1. 6435 X 10 -3 The weight of Ca. CO 3/ g 0. 1645 The % of Ca. CO 3 in eggshell 82. 25%

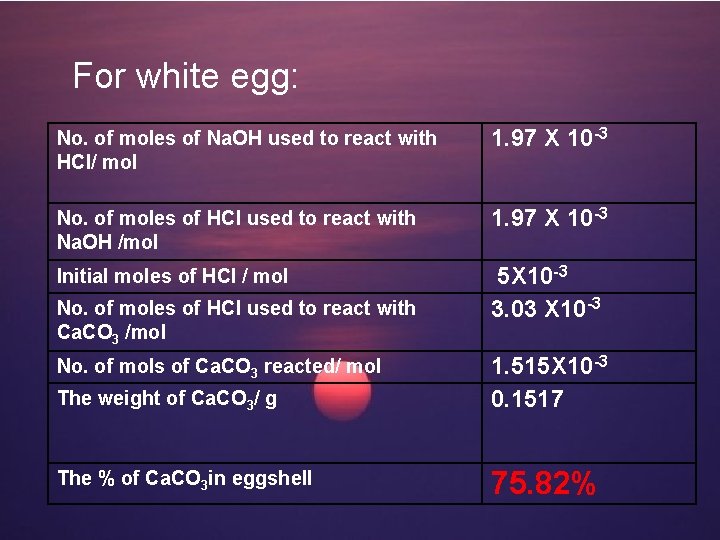
For white egg: No. of moles of Na. OH used to react with HCl/ mol 1. 97 X 10 -3 No. of moles of HCl used to react with Na. OH /mol 1. 97 X 10 -3 Initial moles of HCl / mol 5 X 10 -3 3. 03 X 10 -3 No. of moles of HCl used to react with Ca. CO 3 /mol The weight of Ca. CO 3/ g 1. 515 X 10 -3 0. 1517 The % of Ca. CO 3 in eggshell 75. 82% No. of mols of Ca. CO 3 reacted/ mol

Sources of errors • The eggshell powder did not dissolve completely • Variation in visual judgement at the end point • Instrumental errors of the electronic balance • The eggshell was not fully dried • Some droplets of solution may still adhere on the beaker and the glass rod which lead to the reduction in number of moles of excess HCl we should wash our hands after the experiment • There was vaporization during boiling.

Remarks • During titration, control the stopcock of the burette with your left hand. Swirl the conical flask with your right hand • Use a conical flask to dissolved the eggshell since colourless gas bubbles will be evolved. It is to prevent the solution from jumping out. (CO 2 is formed) • When dissolving the eggshell in a conical flask, use a stirrer to stir the solution well in order to make sure that the eggshell is totally dissolved • After pouring the solution mixture of eggshell and HCl into a volumetric flask, remember to rinse the stirrer and the conical flask with distilled water and pour the washing to the volumetric flask • After filling up the burette with Na. OH, we should remember to remove the filter funnel on the top of the burette • Bubble in the burette should be removed before the initial reading of the burette is read • we should swirl the conical flask after each addition. Distilled water should be added to rinse the Na. OH down the flask • Put a white tile under the conical flask for clearer observation

Safety precautions • Dilute Na. OH is corrosive • Do not touch chemicals with bare hands • Safety goggles should be put on during the experiment • We should wash our hands after the experiment

Conclusion • By comparing the percentage of two eggs, brown egg (Malaysia) has a higher percentage than white egg (USA) indicate brown egg has a higher strength than white egg. It also show that eggs from USA have a more serious problem of using DDT.

Group members • Hung Ching Yin (4) Took photos, Typing, Search for information, Calculations, Grouping information • Wai Sze Wah (8) Typing, Search for information, Calculations • Poon Yee Man (7) Record the results, typing, search for information, Power. Point Design • Lin Yin Shing (23) Typing, search for information • Yuen Chun Fai (30) Typing, search for information, Caclculations

Sources of references • http: //www. chem. csustan. edu/chem 1102/E gg. htm • www. wikipedia. com

The end! Thank you for your attention! Bye!