Chemistry of NOx and SOA VOC Oxidation by

Chemistry of NOx and SOA: VOC Oxidation by Nitrate Radicals Andrew Rollins Cohen research group, department of chemistry University of California, Berkeley, USA
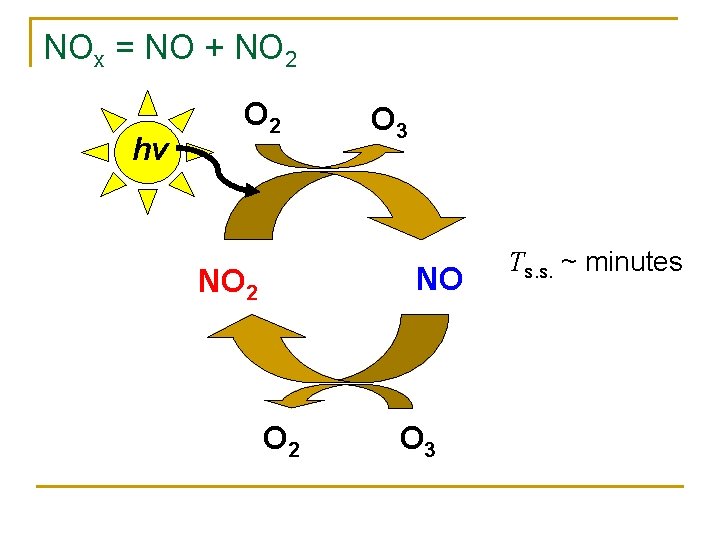
NOx = NO + NO 2 hν O 2 O 3 NO NO 2 O 3 Τs. s. ~ minutes
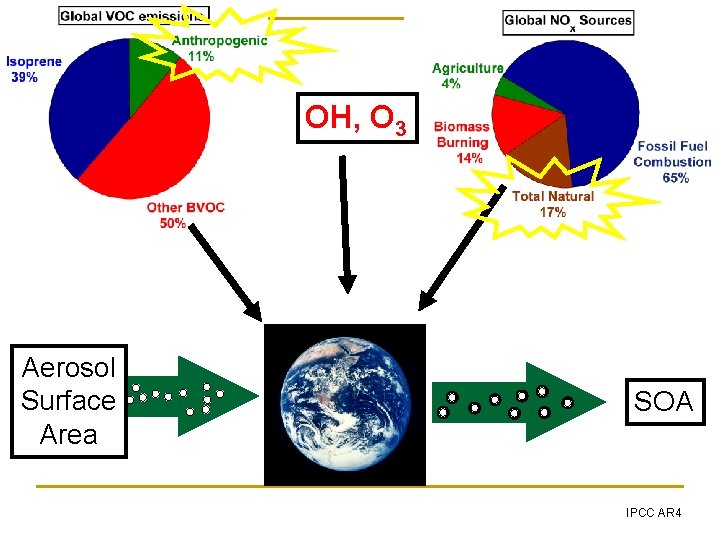
OH, O 3 Aerosol Surface Area SOA IPCC AR 4
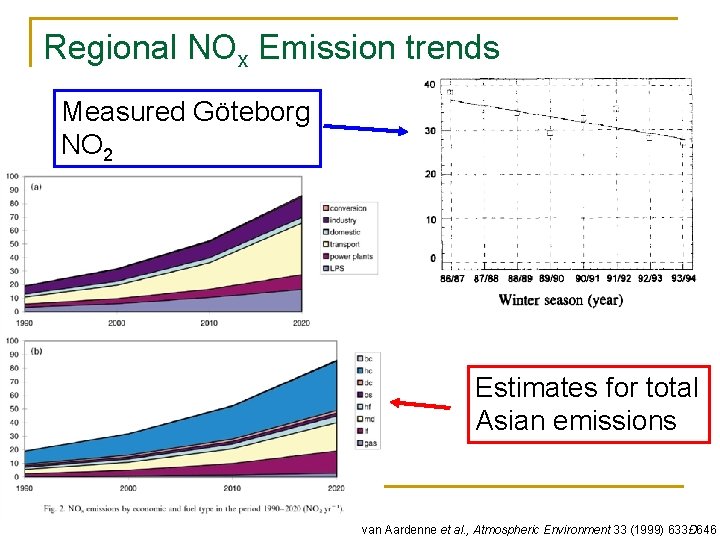
Regional NOx Emission trends Measured Göteborg NO 2 Estimates for total Asian emissions van Aardenne et al. , Atmospheric Environment 33 (1999) 633Ð 646

outline n Motivations q q n n Global/Regional changes in NOx: VOC emissions NOx emissions as control strategy 2 classes of NOx effects on SOA production q Product distributions / RO 2 chemistry q NO 3 + VOC → SOA Nitrate Radical (NO 3) Isoprene + NO 3 SAPHIR experiment Alkyl Nitrate kinetic uptake experiments
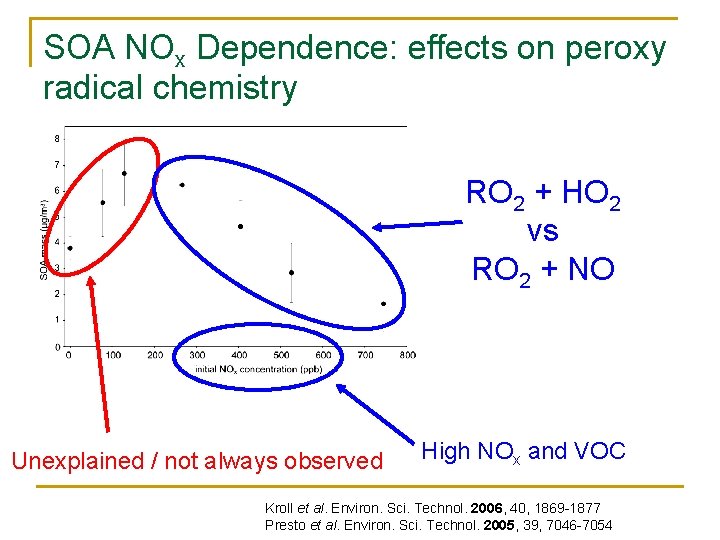
SOA NOx Dependence: effects on peroxy radical chemistry RO 2 + HO 2 vs RO 2 + NO Unexplained / not always observed High NOx and VOC Kroll et al. Environ. Sci. Technol. 2006, 40, 1869 -1877 Presto et al. Environ. Sci. Technol. 2005, 39, 7046 -7054
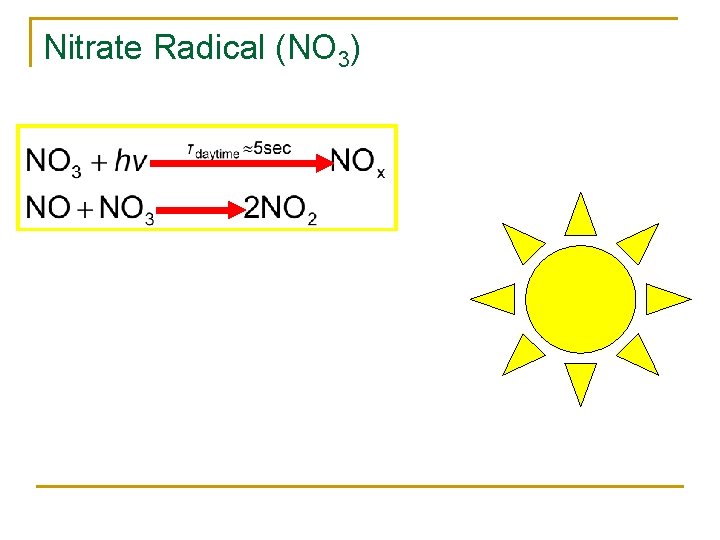
Nitrate Radical (NO 3)
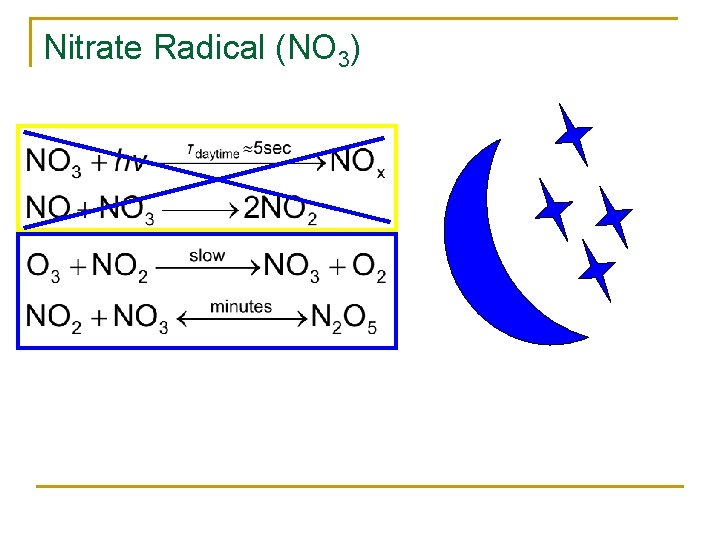
Nitrate Radical (NO 3)
![Nitrate Radical (NO 3) Sunset [NO 3]≈10’s ppt Brown et al 2004 Nitrate Radical (NO 3) Sunset [NO 3]≈10’s ppt Brown et al 2004](http://slidetodoc.com/presentation_image/eccb9930b059fa2dbbf107eefd2a8e90/image-9.jpg)
Nitrate Radical (NO 3) Sunset [NO 3]≈10’s ppt Brown et al 2004

NO 3 vs OH and O 3 as VOC sinks VOC k. OH k. O 3 k. NO 3 Isoprene 102 1. 28 e-5 0. 68 α-pinene 54 8. 5 e-5 6. 2 Limonene 170 2. 0 e-4 12 Methacrolein 34 1. 1 e-6 4. 4 e-3 0. 5 x 107 cm-3 = 0. 2 ppt OH 20 ppt NO 3 Brown et al 2004
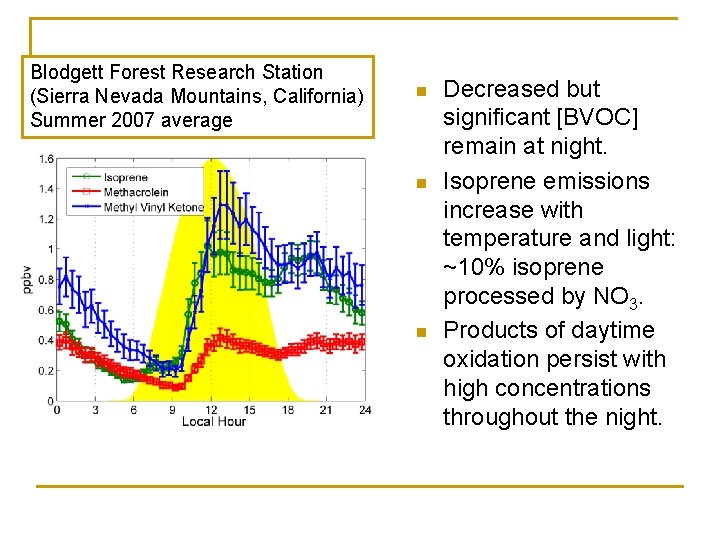
Blodgett Forest Research Station (Sierra Nevada Mountains, California) Summer 2007 average n n n Decreased but significant [BVOC] remain at night. Isoprene emissions increase with temperature and light: ~10% isoprene processed by NO 3. Products of daytime oxidation persist with high concentrations throughout the night.
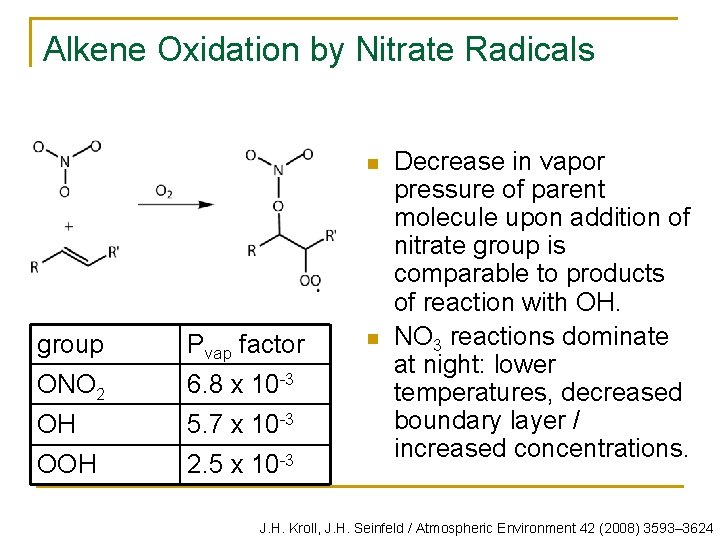
Alkene Oxidation by Nitrate Radicals n group Pvap factor ONO 2 6. 8 x 10 -3 OH 5. 7 x 10 -3 OOH 2. 5 x 10 -3 n Decrease in vapor pressure of parent molecule upon addition of nitrate group is comparable to products of reaction with OH. NO 3 reactions dominate at night: lower temperatures, decreased boundary layer / increased concentrations. J. H. Kroll, J. H. Seinfeld / Atmospheric Environment 42 (2008) 3593– 3624
Jϋlich chamber experiments n n SAPHIR chamber ~ 260 m 3. Near Ambient NOx & VOC Long chamber runs (> 12 hours) NO 3 SOA experiments: q q q Linomene Β-Pinene (high and low RH) Isoprene (seeded)

Isoprene + NO 3 n n n 15 hour run Max 10 ppb isoprene, 30 ppb NO 2, 60 ppb O 3 NH 3(SO 4)2 seed AMS, SMPS, PTRMS, GC, TDLIF Many NO 3 / N 2 O 5 measurements
Isoprene C 5 H 8 n n n 440 -6601 Tg. C / ~13002 Tg. C total non-methane VOC (biogenic + anthropogenic) ≈ 34 – 50% total carbon. Two double bonds/ multiple oxidation steps / high reactivity to OH, O 3, NO 3. Isoprene SOA potential is poorly understood, small yields of SOA (5% by NO 3) could be large Fractions of total global SOA annual production (2 -3 Tg. C / 12 -70 Tg. C)4 Early OH and O 3 experiments (100 s of ppbs isoprene and NOx) concluded Isoprene not an SOA precursor, because 1 st generation oxidation products of isoprene are too volatile. More recently photochemical experiments demonstrate that Isoprene possibly contributes up to 47%5 of global SOA, by polymerization and heterogeneous chemistry of initial oxidation products Alkyl Nitrate formation by addition of NO 3 observed with high (80%) yields, increase MW and adding functionality. SOA yields reported at 4. 3% - 23. 8% (increasing with existing OM). 6 1 Guenther et al. 2006 2 Goldstein and Galbally 2007 3 Calvert et al. 2000 4 Kanakidou et al. 2005 et al. 2007 6 Ng et al. 2008 5 Zhang
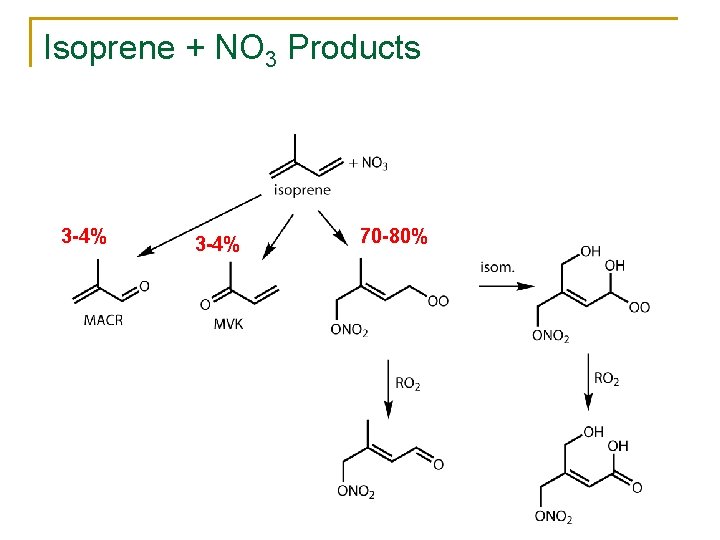
Isoprene + NO 3 Products 3 -4% 70 -80%
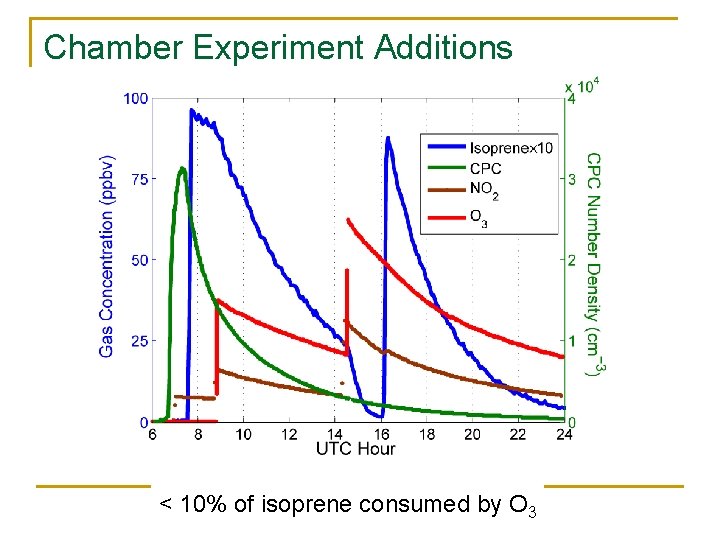
Chamber Experiment Additions < 10% of isoprene consumed by O 3
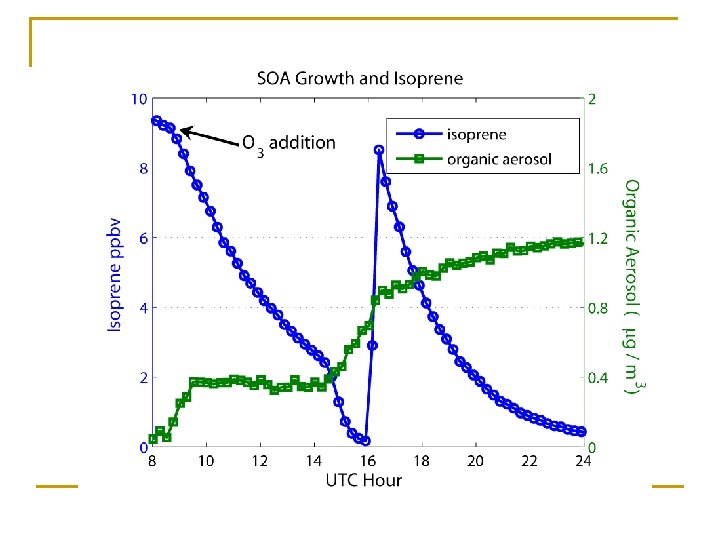
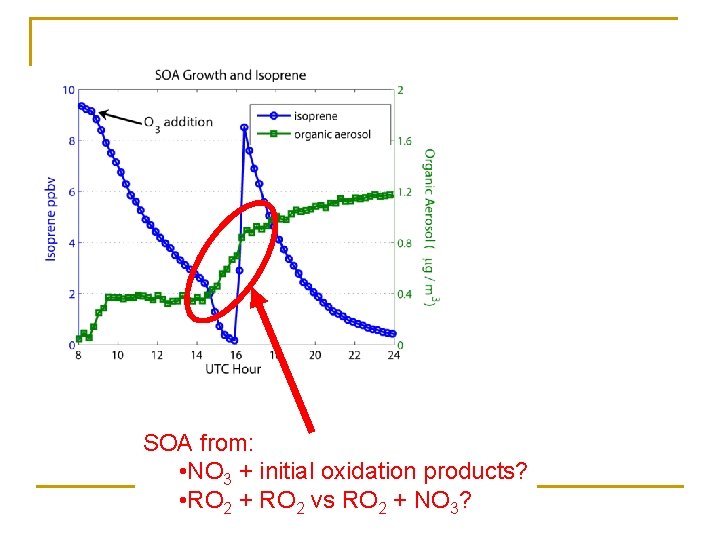
SOA from: • NO 3 + initial oxidation products? • RO 2 + RO 2 vs RO 2 + NO 3?
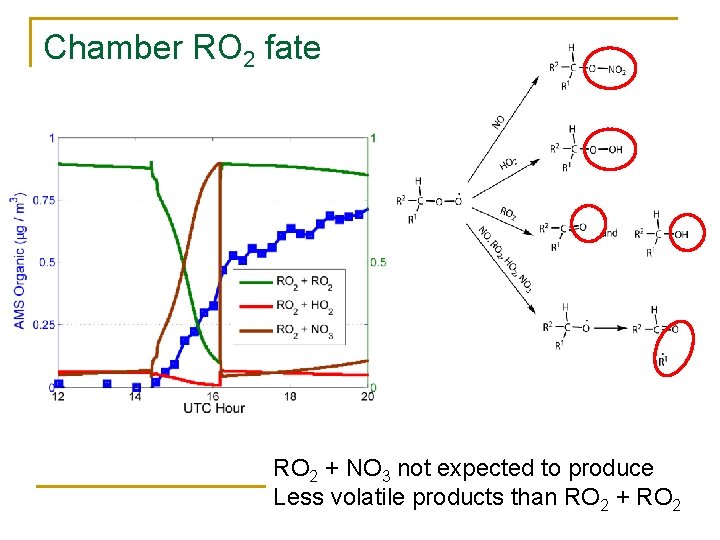
Chamber RO 2 fate RO 2 + NO 3 not expected to produce Less volatile products than RO 2 + RO 2
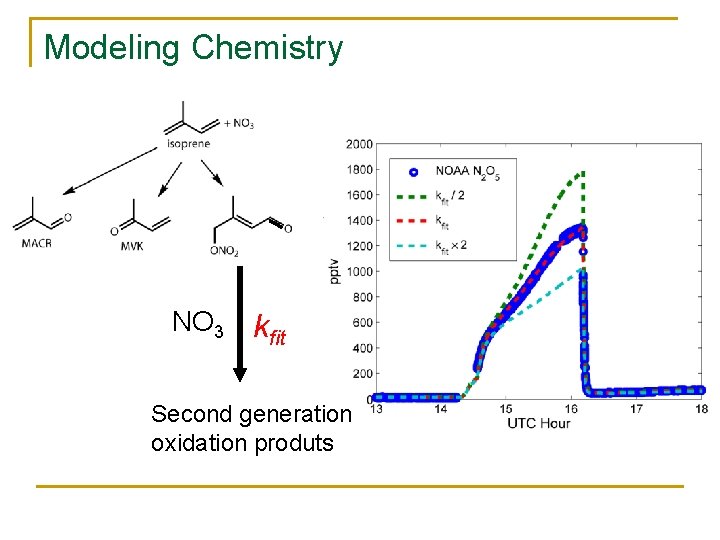
Modeling Chemistry NO 3 kfit Second generation oxidation produts
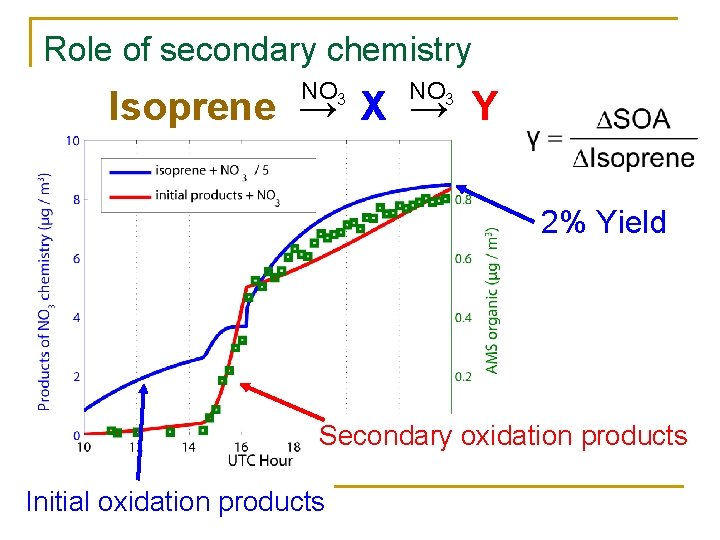
Role of secondary chemistry NO 3 Isoprene → X → Y 2% Yield Secondary oxidation products Initial oxidation products
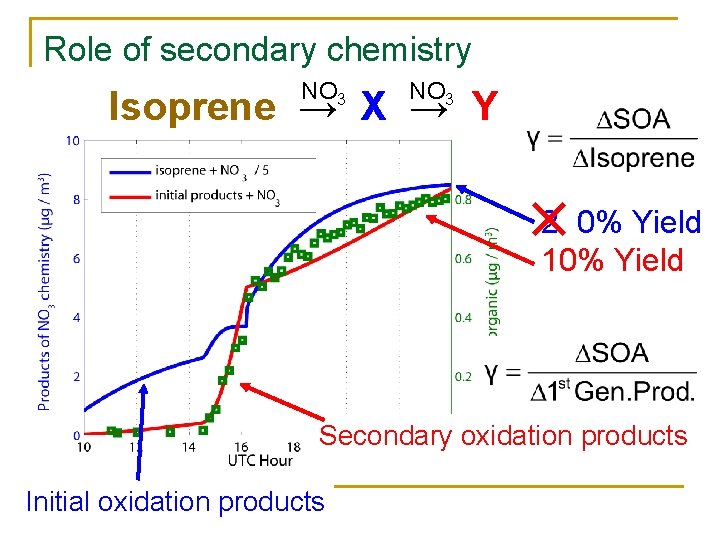
Role of secondary chemistry NO 3 Isoprene → X → Y 2 0% Yield 10% Yield Secondary oxidation products Initial oxidation products
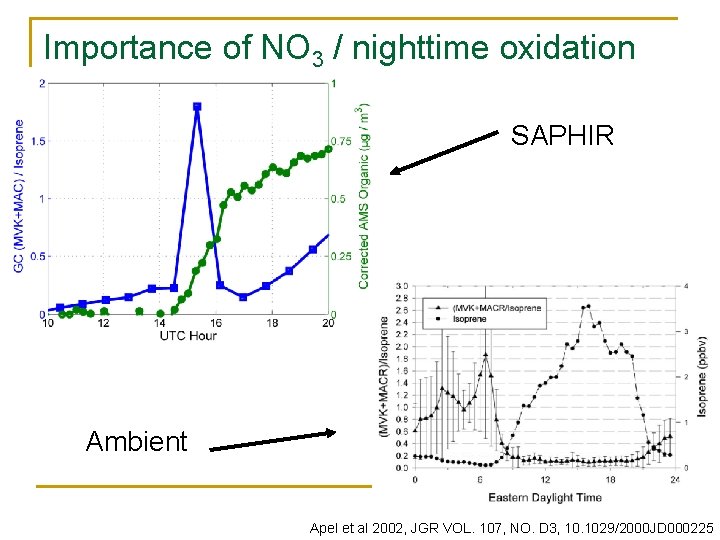
Importance of NO 3 / nighttime oxidation SAPHIR Ambient Apel et al 2002, JGR VOL. 107, NO. D 3, 10. 1029/2000 JD 000225
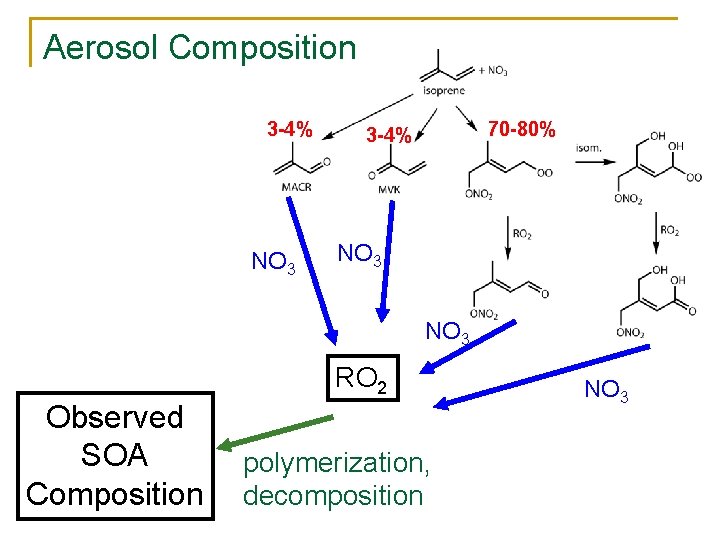
Aerosol Composition 3 -4% NO 3 70 -80% 3 -4% NO 3 RO 2 Observed SOA Composition polymerization, decomposition NO 3
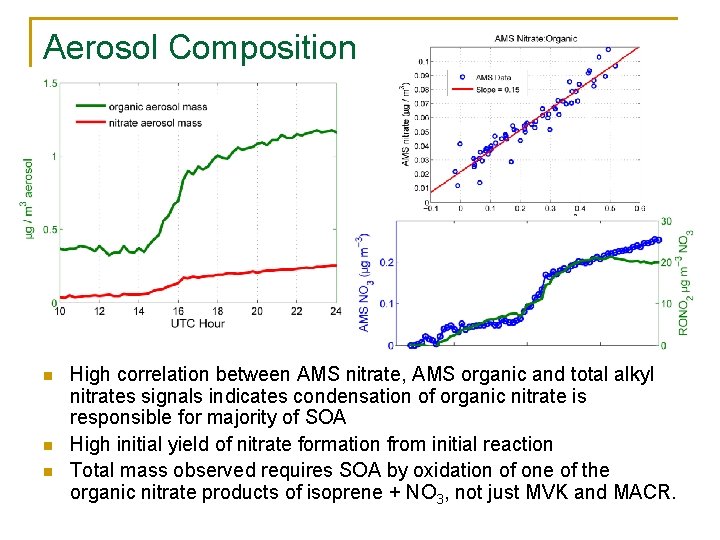
Aerosol Composition n High correlation between AMS nitrate, AMS organic and total alkyl nitrates signals indicates condensation of organic nitrate is responsible for majority of SOA High initial yield of nitrate formation from initial reaction Total mass observed requires SOA by oxidation of one of the organic nitrate products of isoprene + NO 3, not just MVK and MACR.
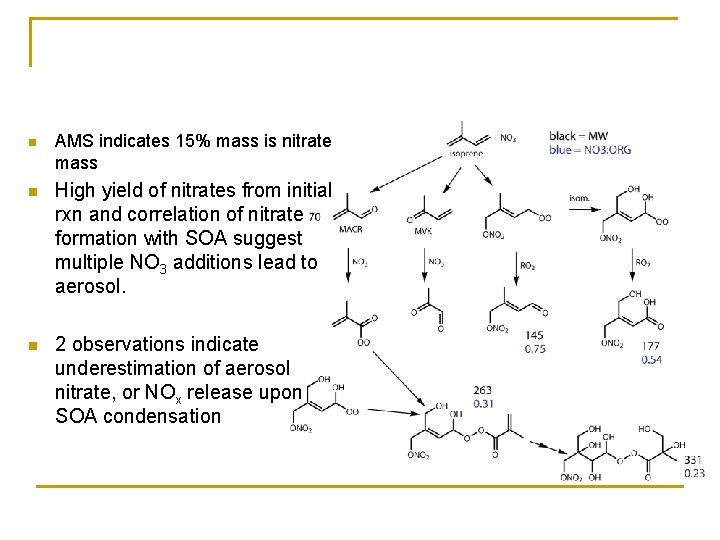
n AMS indicates 15% mass is nitrate mass n High yield of nitrates from initial rxn and correlation of nitrate formation with SOA suggest multiple NO 3 additions lead to aerosol. n 2 observations indicate underestimation of aerosol nitrate, or NOx release upon SOA condensation
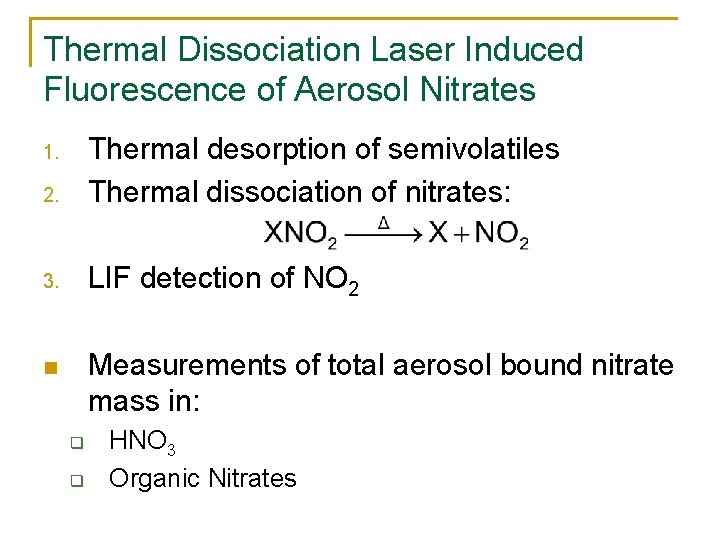
Thermal Dissociation Laser Induced Fluorescence of Aerosol Nitrates 2. Thermal desorption of semivolatiles Thermal dissociation of nitrates: 3. LIF detection of NO 2 n Measurements of total aerosol bound nitrate mass in: 1. q q HNO 3 Organic Nitrates
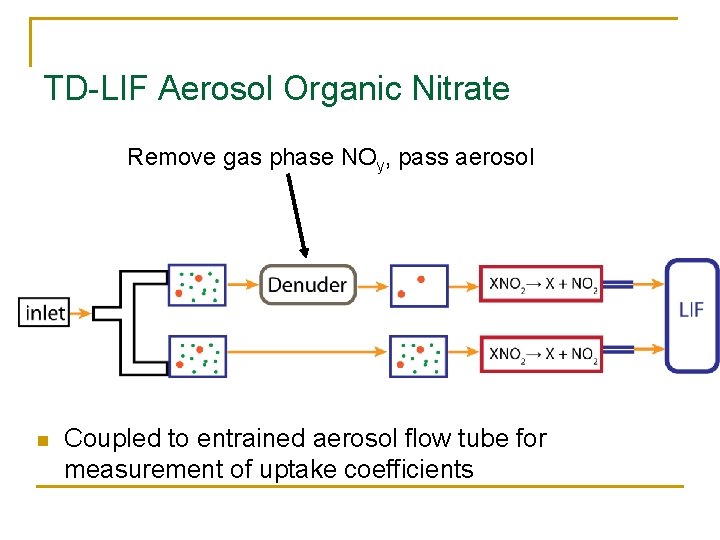
TD-LIF Aerosol Organic Nitrate Remove gas phase NOy, pass aerosol n Coupled to entrained aerosol flow tube for measurement of uptake coefficients
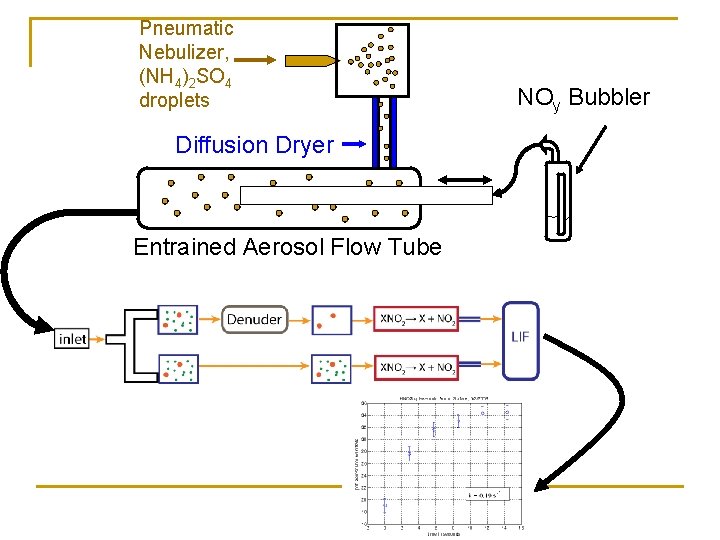
Pneumatic Nebulizer, (NH 4)2 SO 4 droplets Diffusion Dryer Entrained Aerosol Flow Tube NOy Bubbler
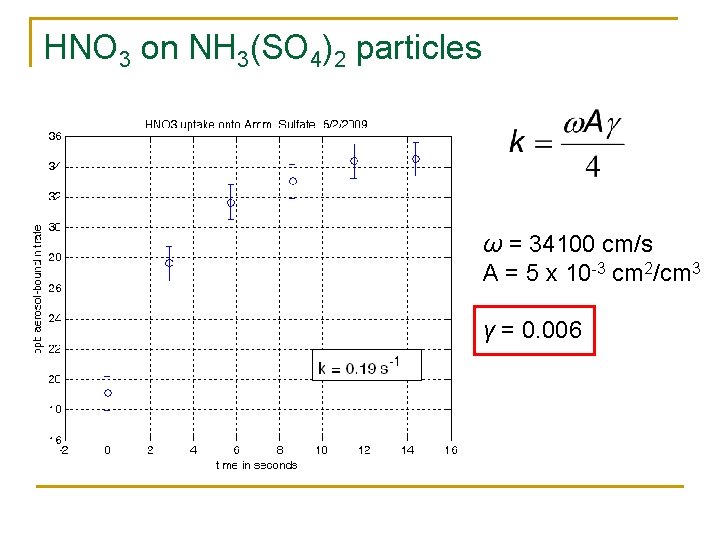
HNO 3 on NH 3(SO 4)2 particles ω = 34100 cm/s A = 5 x 10 -3 cm 2/cm 3 γ = 0. 006
Uptake of synthesized organic nitrates • Salts • Organic particles

NOx / Aerosol Research Questions n n Effects of changing NOx / VOC emissions on the total SOA production, and speciation. q Total yield changes? q Aerosol composition? If composition, is CCN affected? Current research: q Chamber SOA and organic nitrate aerosol yields / mechanisms from NO 3 oxidation of BVOC’s. q Flow tube uptake measurements of organic nitrates / nitric acid on aerosol surfaces.
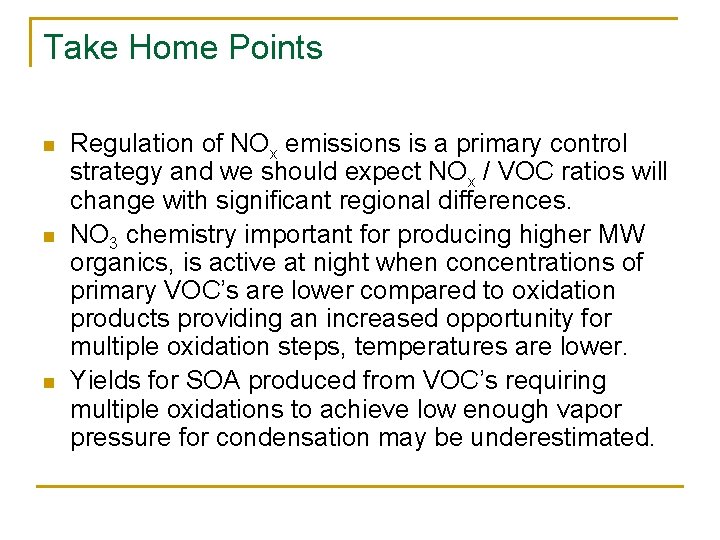
Take Home Points n n n Regulation of NOx emissions is a primary control strategy and we should expect NOx / VOC ratios will change with significant regional differences. NO 3 chemistry important for producing higher MW organics, is active at night when concentrations of primary VOC’s are lower compared to oxidation products providing an increased opportunity for multiple oxidation steps, temperatures are lower. Yields for SOA produced from VOC’s requiring multiple oxidations to achieve low enough vapor pressure for condensation may be underestimated.

Thanks to… n n n Cohen Group q Juliane Fry (Reed College, Oregon) q Ronald Cohen q Paul Wooldridge F. Z. Jϋlich scientists q Astrid Kiendler. Scharr Steve Brown, Hendrik Fuchs, Bill Dubé (NOAA) n Sarpong Group (UCB) q Walter Singaram q Massoud Motamed
- Slides: 35