Chemistry of Nitrogencontaining Organic Compounds FSF Full Structural







- Slides: 22

Chemistry of Nitrogen-containing Organic Compounds FSF = Full Structural Formula

Key Areas • Functional group – name and structure • Characteristic chemical reactions - type - reactants - products - conditions - mechanism

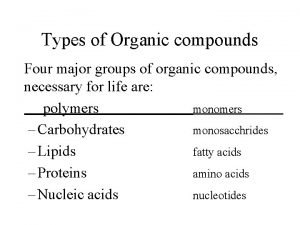
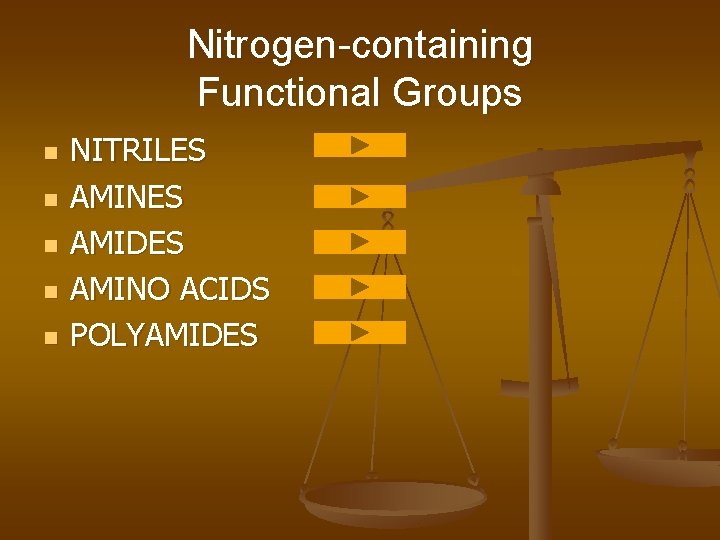
Nitrogen-containing Functional Groups n n n NITRILES AMINES AMIDES AMINO ACIDS POLYAMIDES

NITRILES R C N • Not to be confused with cyanides (CN- ) • First member is ethanenitrile. Draw the full structural formula. • Name the fourth member of the family. • C N very polar • Will the C-atom be a nucleophile or electrophile?

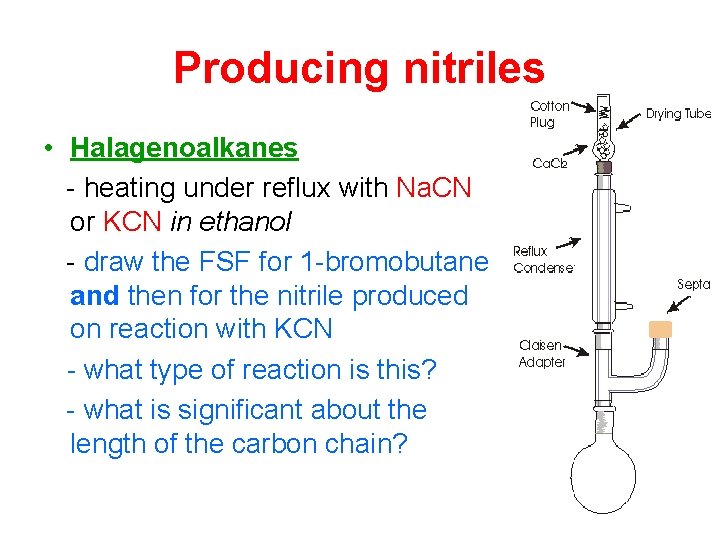
Producing nitriles • Halagenoalkanes - heating under reflux with Na. CN or KCN in ethanol - draw the FSF for 1 -bromobutane and then for the nitrile produced on reaction with KCN - what type of reaction is this? - what is significant about the length of the carbon chain?

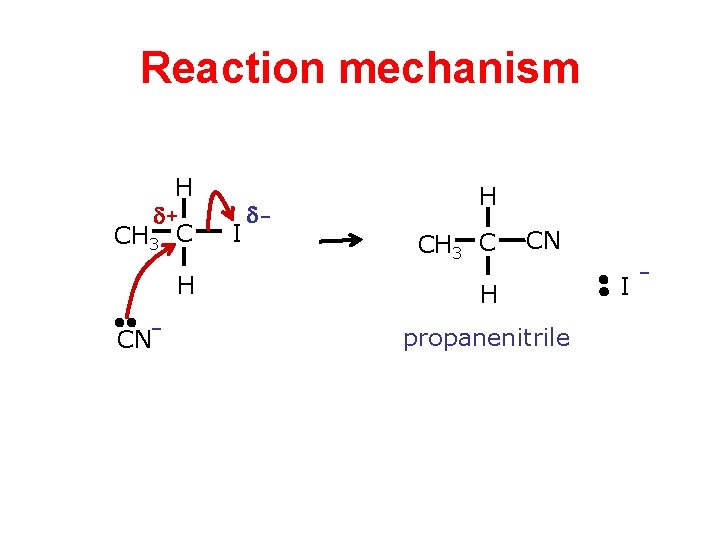
Reaction mechanism H + CH 3 C H CN- I - H CH 3 C CN H propanenitrile I -

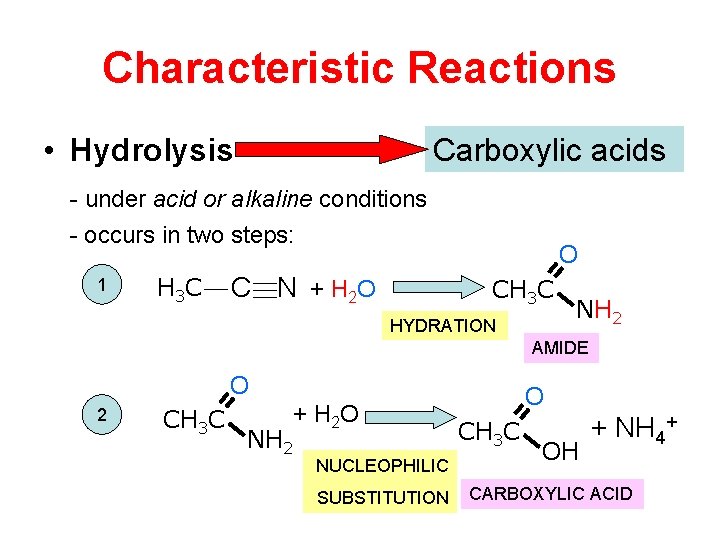
Characteristic Reactions • Hydrolysis Carboxylic acids - under acid or alkaline conditions - occurs in two steps: 1 H 3 C O C N + H 2 O CH 3 C HYDRATION NH 2 AMIDE O 2 CH 3 C + H 2 O NH 2 NUCLEOPHILIC SUBSTITUTION O CH 3 C OH + NH 4+ CARBOXYLIC ACID

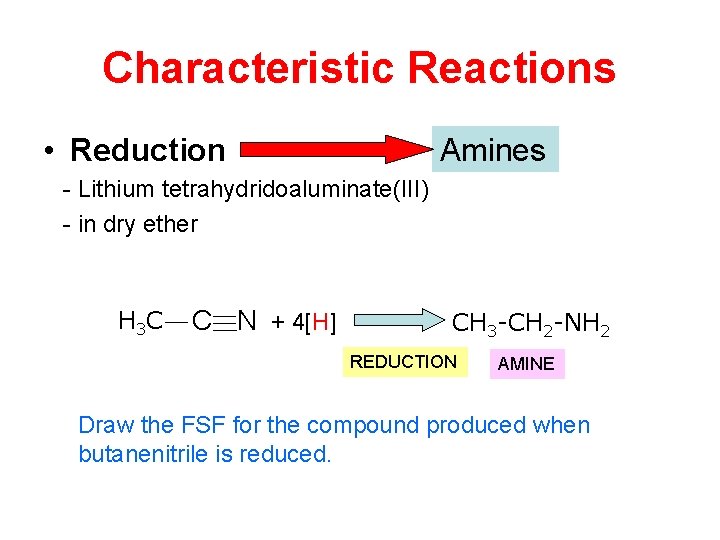
Characteristic Reactions • Reduction Amines - Lithium tetrahydridoaluminate(III) - in dry ether H 3 C C N + 4[H] CH 3 -CH 2 -NH 2 REDUCTION AMINE Draw the FSF for the compound produced when butanenitrile is reduced.

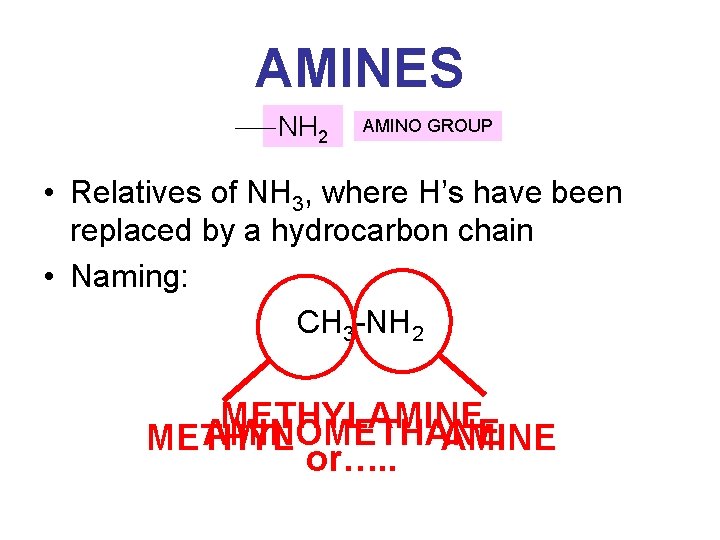
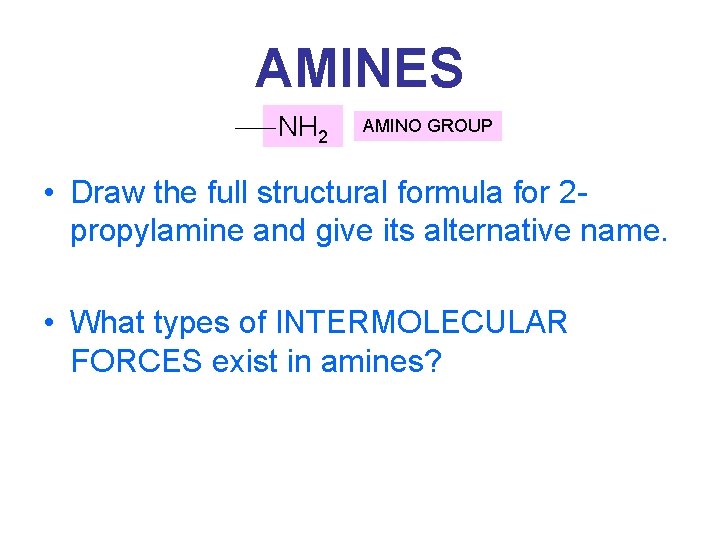
AMINES NH 2 AMINO GROUP • Relatives of NH 3, where H’s have been replaced by a hydrocarbon chain • Naming: CH 3 -NH 2 METHYLAMINE AMINOMETHANE METHYL AMINE or…. .

AMINES NH 2 AMINO GROUP • Draw the full structural formula for 2 propylamine and give its alternative name. • What types of INTERMOLECULAR FORCES exist in amines?

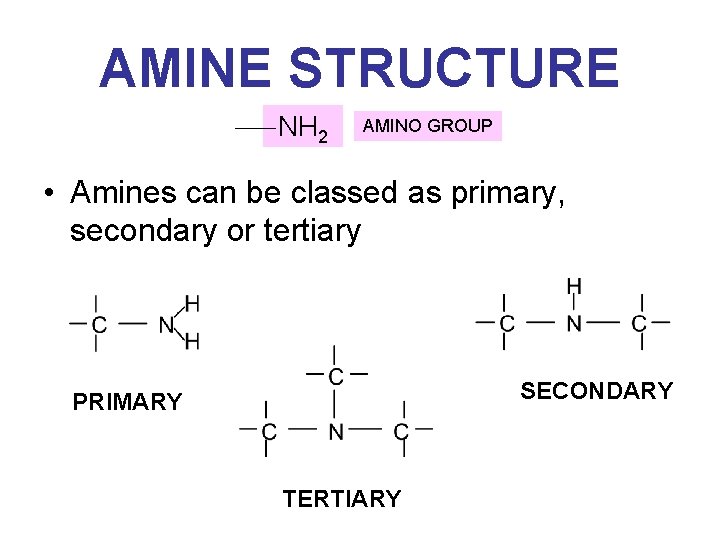
AMINE STRUCTURE NH 2 AMINO GROUP • Amines can be classed as primary, secondary or tertiary SECONDARY PRIMARY TERTIARY

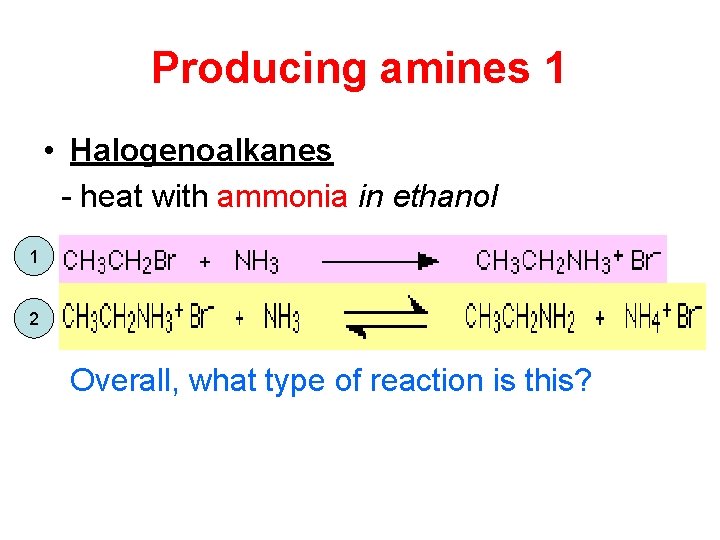
Producing amines 1 • Halogenoalkanes - heat with ammonia in ethanol 1 2 Overall, what type of reaction is this?

Producing amines 2 • Nitriles (What is a nitrile? ) -C≡N - reduction - using Li. Al. H 4 (in dry ether + water) • Draw the simplified strucural formula for the amine produced by reducing butanenitrile.

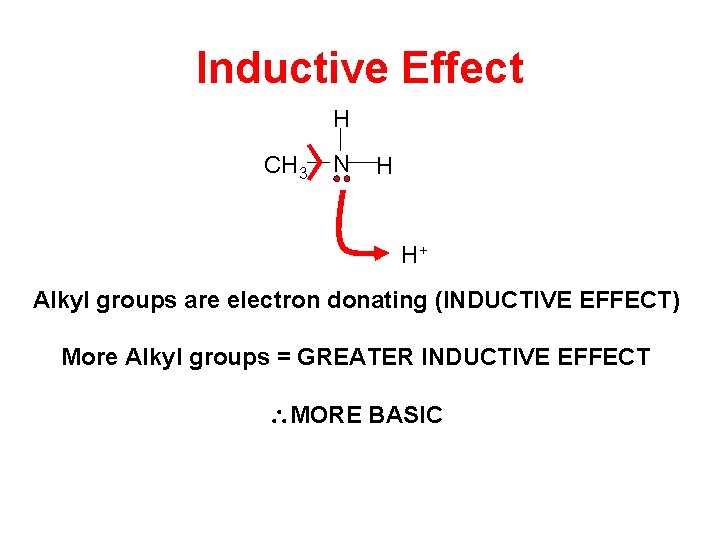
Reactions of amines H H N H • As bases Primary amines, although weak bases, are stronger than ammonia Explained by the inductive effect

Inductive Effect H CH 3 N H H+ Alkyl groups are electron donating (INDUCTIVE EFFECT) More Alkyl groups = GREATER INDUCTIVE EFFECT MORE BASIC

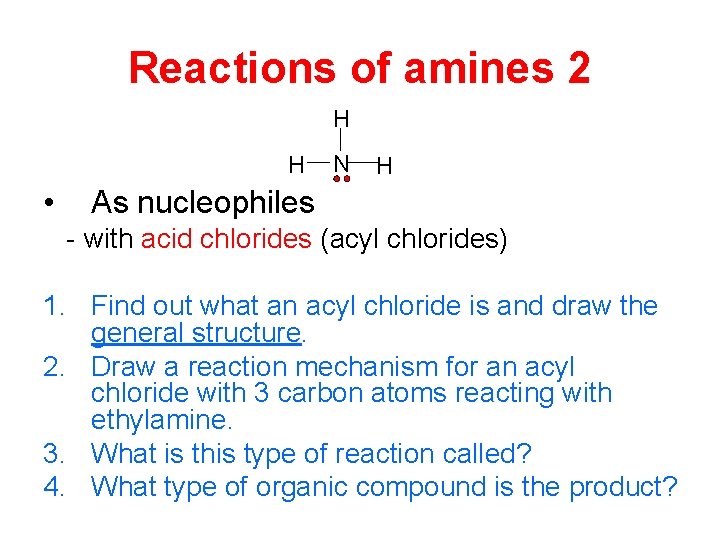
Reactions of amines 2 H H • N H As nucleophiles - with acid chlorides (acyl chlorides) 1. Find out what an acyl chloride is and draw the general structure. 2. Draw a reaction mechanism for an acyl chloride with 3 carbon atoms reacting with ethylamine. 3. What is this type of reaction called? 4. What type of organic compound is the product?

AMIDES O R-C NH 2 • Are white crystalline solids at room temperature (expect methanamide) • Are important in polymer chemistry • How can amides be made in the lab?

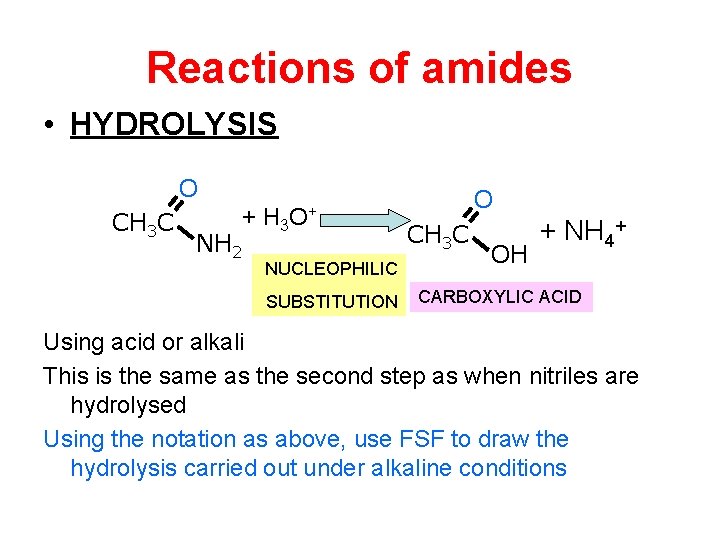
Reactions of amides • HYDROLYSIS O CH 3 C + H 3 NH 2 O+ NUCLEOPHILIC SUBSTITUTION O CH 3 C OH + NH 4+ CARBOXYLIC ACID Using acid or alkali This is the same as the second step as when nitriles are hydrolysed Using the notation as above, use FSF to draw the hydrolysis carried out under alkaline conditions

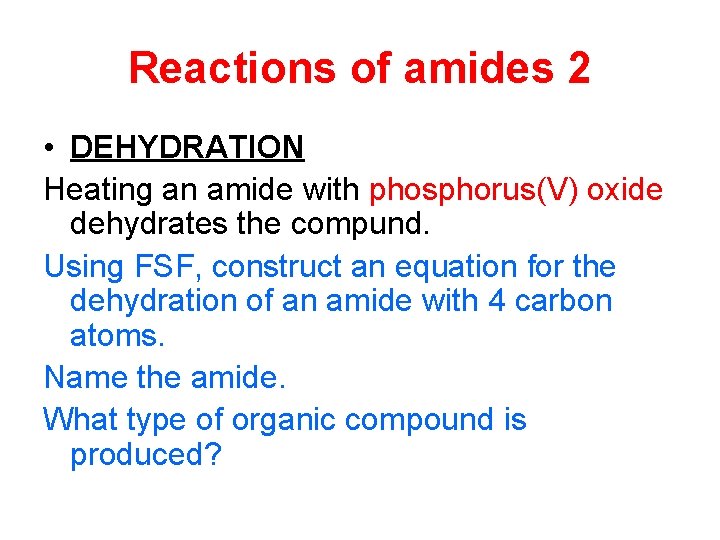
Reactions of amides 2 • DEHYDRATION Heating an amide with phosphorus(V) oxide dehydrates the compund. Using FSF, construct an equation for the dehydration of an amide with 4 carbon atoms. Name the amide. What type of organic compound is produced?

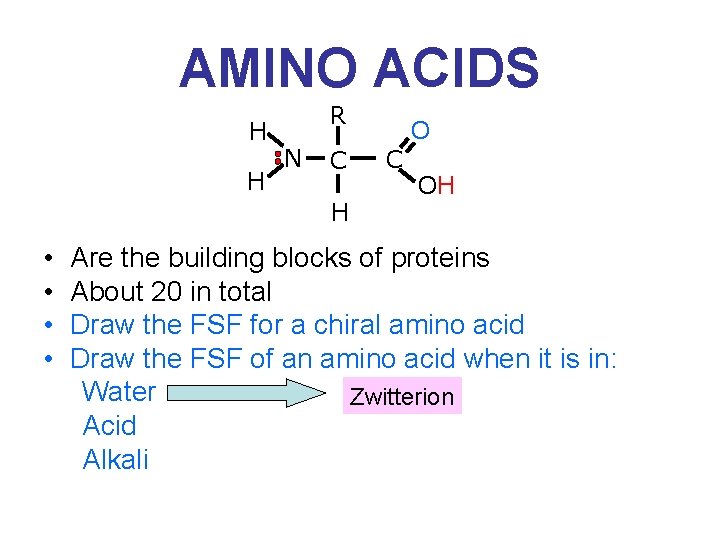
AMINO ACIDS H H R N C H • • O C OH Are the building blocks of proteins About 20 in total Draw the FSF for a chiral amino acid Draw the FSF of an amino acid when it is in: Water Zwitterion Acid Alkali

Reactions of amino acids • Amino acids join together to form peptides • This is a condensation reaction R H O N C R 1 amide An AMIDE group Draw the FSF of a peptide consisting of alanine, cysteine and serine

POLYAMIDES O HO • • C R C O OH H H N R N H Formed from a diamine and a diacid Nylon is a polyamide Kevlar is an aramid Used to make bullet-proof vests H
 Priority order of functional groups
Priority order of functional groups Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry How are water-soluble vitamins absorbed
How are water-soluble vitamins absorbed Vitamin classification chart
Vitamin classification chart Types of organic compound
Types of organic compound Decomposition of organic compounds
Decomposition of organic compounds Organic and inorganic compounds experiment
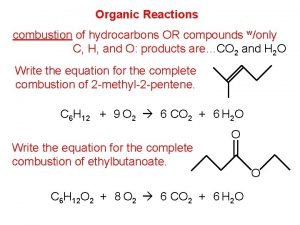
Organic and inorganic compounds experiment Organic combustion
Organic combustion These are organic compounds made by living things
These are organic compounds made by living things All organic compounds must contain the element
All organic compounds must contain the element Organic compounds must contain
Organic compounds must contain Intro to organic chemistry
Intro to organic chemistry Difference between organic and inorganic
Difference between organic and inorganic Purification and characterization of organic compounds
Purification and characterization of organic compounds Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Organic vs inorganic compounds
Organic vs inorganic compounds What is the classification of organic compounds
What is the classification of organic compounds Group of carbon
Group of carbon Principle of simple distillation
Principle of simple distillation Reactions of organic compounds
Reactions of organic compounds Organic compounds grade 10 life science
Organic compounds grade 10 life science Organic halogen compounds
Organic halogen compounds