CHEMISTRY OF NATURAL PRODUCTS CHEM 445 Credit 21


- Slides: 23

CHEMISTRY OF NATURAL PRODUCTS CHEM 445 Credit (2+1) Chemistry Department Chemistry College of of Science College Science Dr. Assem Barakat, Ph. D Associate Professor of Organic Chemistry Email: ambarakat@ksu. edu. sa

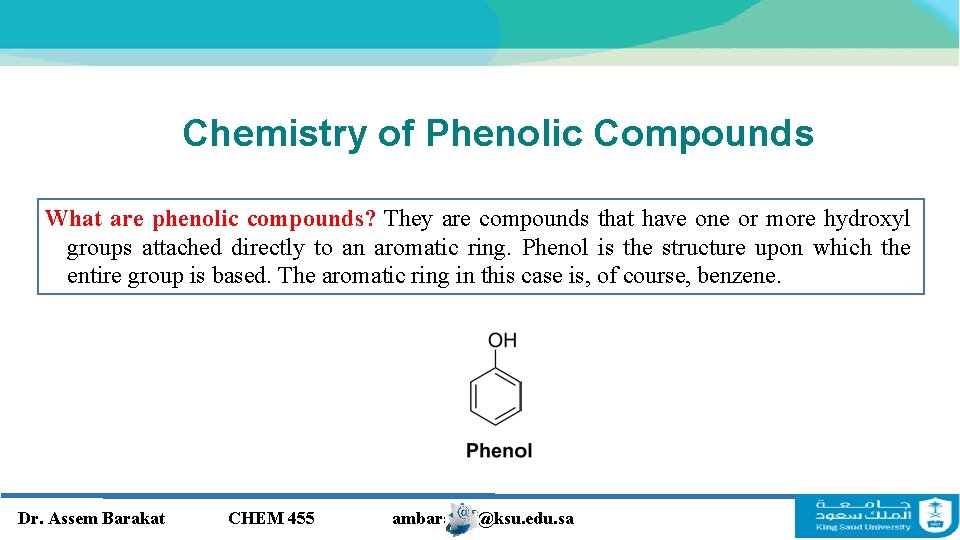
Chemistry of Phenolic Compounds What are phenolic compounds? They are compounds that have one or more hydroxyl groups attached directly to an aromatic ring. Phenol is the structure upon which the entire group is based. The aromatic ring in this case is, of course, benzene. Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

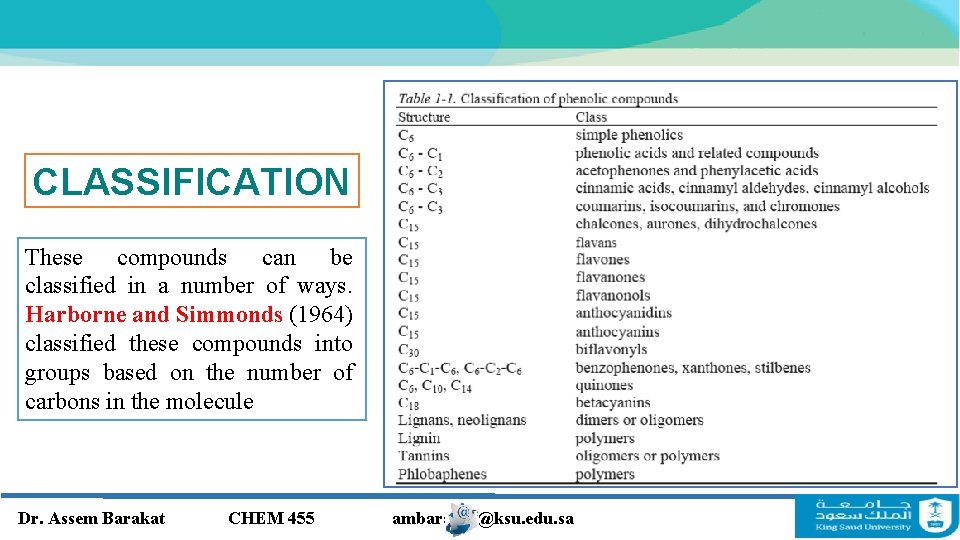
CLASSIFICATION These compounds can be classified in a number of ways. Harborne and Simmonds (1964) classified these compounds into groups based on the number of carbons in the molecule Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

An alternative classification has been used by Swain and Bate-Smith (1962). They grouped the phenols in “common” and “less common” categories. Ribéreau-Gayon (1972) grouped the phenols into three families as follows: 1. Widely distributed phenols - ubiquitous to all plants, or of importance in a specific plant 2. Phenols that are less widely distributed - limited number of compounds known 3. Phenolic constituents present as polymers. Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

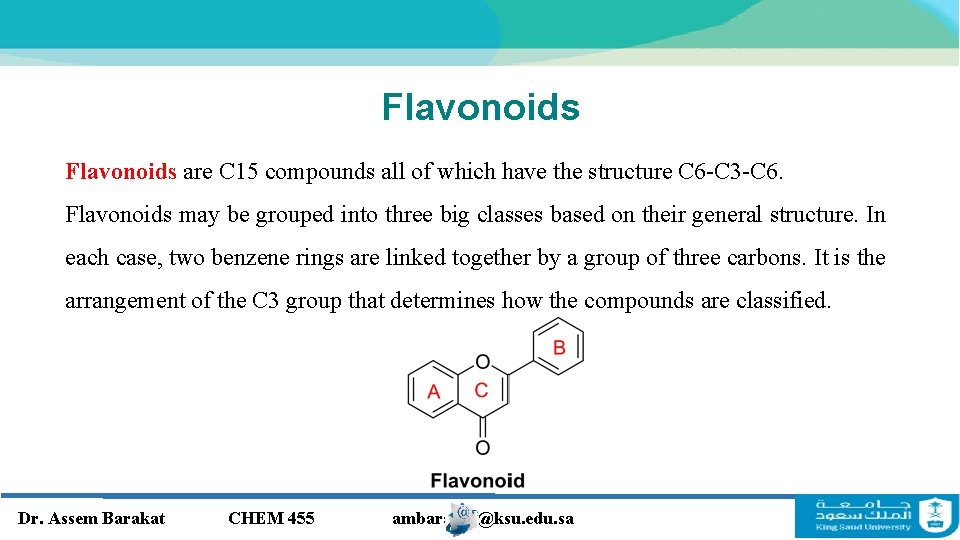
Flavonoids are C 15 compounds all of which have the structure C 6 -C 3 -C 6. Flavonoids may be grouped into three big classes based on their general structure. In each case, two benzene rings are linked together by a group of three carbons. It is the arrangement of the C 3 group that determines how the compounds are classified. Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

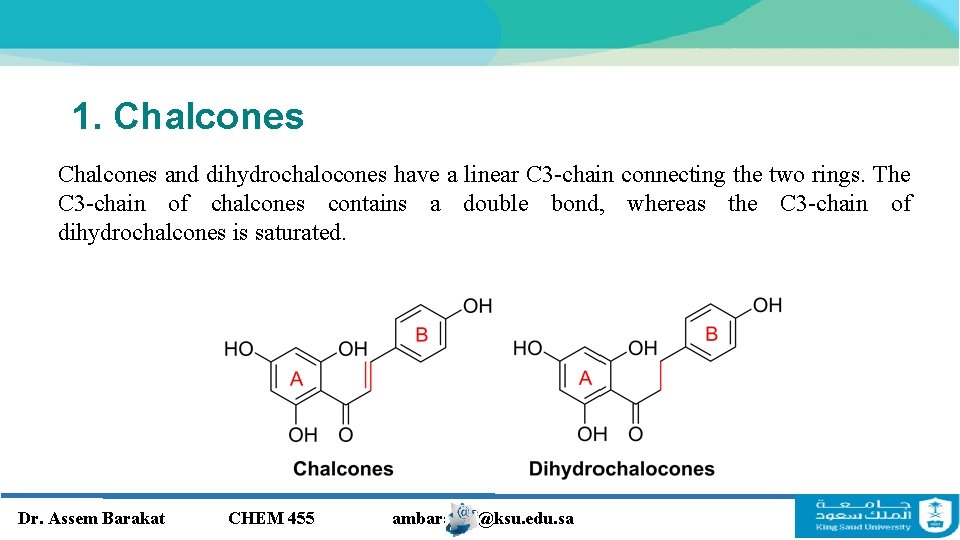
1. Chalcones and dihydrochalocones have a linear C 3 -chain connecting the two rings. The C 3 -chain of chalcones contains a double bond, whereas the C 3 -chain of dihydrochalcones is saturated. Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

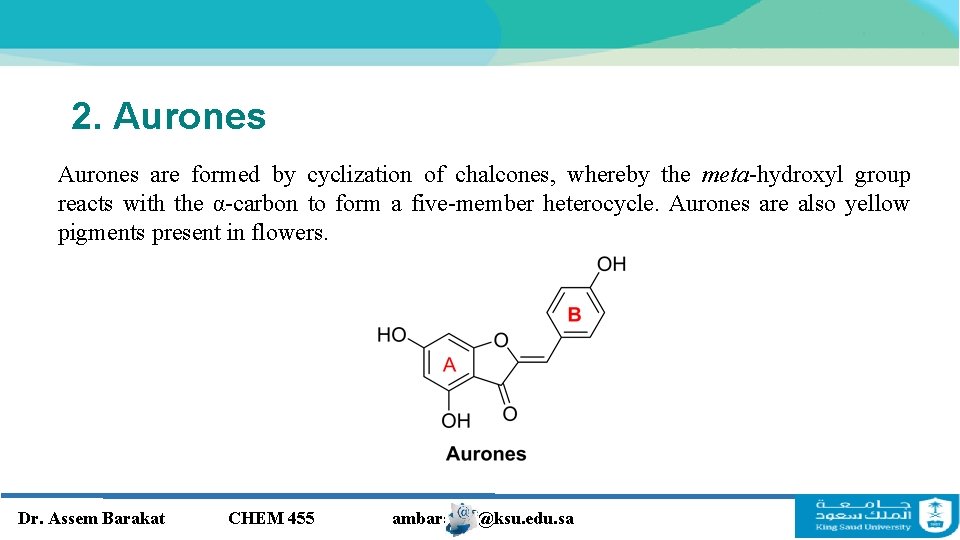
2. Aurones are formed by cyclization of chalcones, whereby the meta-hydroxyl group reacts with the α-carbon to form a five-member heterocycle. Aurones are also yellow pigments present in flowers. Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

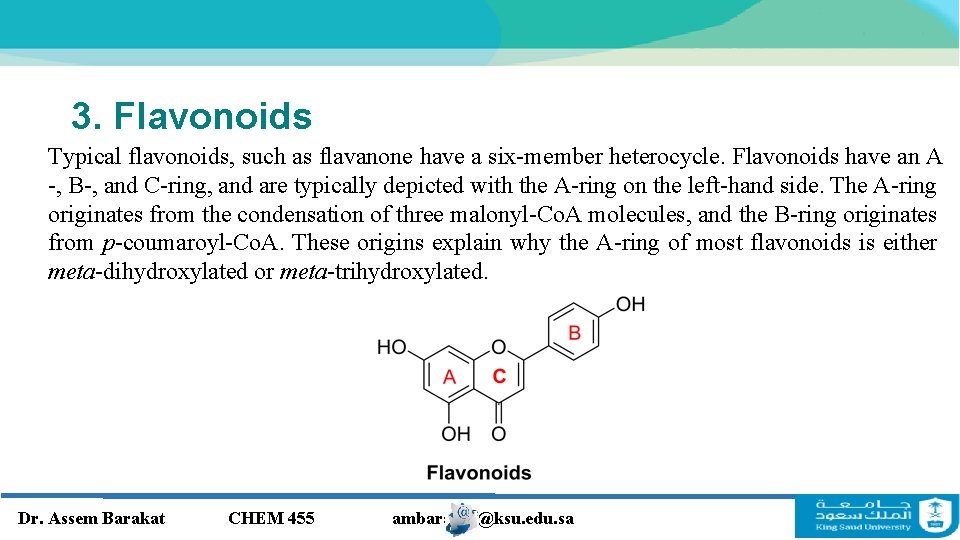
3. Flavonoids Typical flavonoids, such as flavanone have a six-member heterocycle. Flavonoids have an A -, B-, and C-ring, and are typically depicted with the A-ring on the left-hand side. The A-ring originates from the condensation of three malonyl-Co. A molecules, and the B-ring originates from p-coumaroyl-Co. A. These origins explain why the A-ring of most flavonoids is either meta-dihydroxylated or meta-trihydroxylated. Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

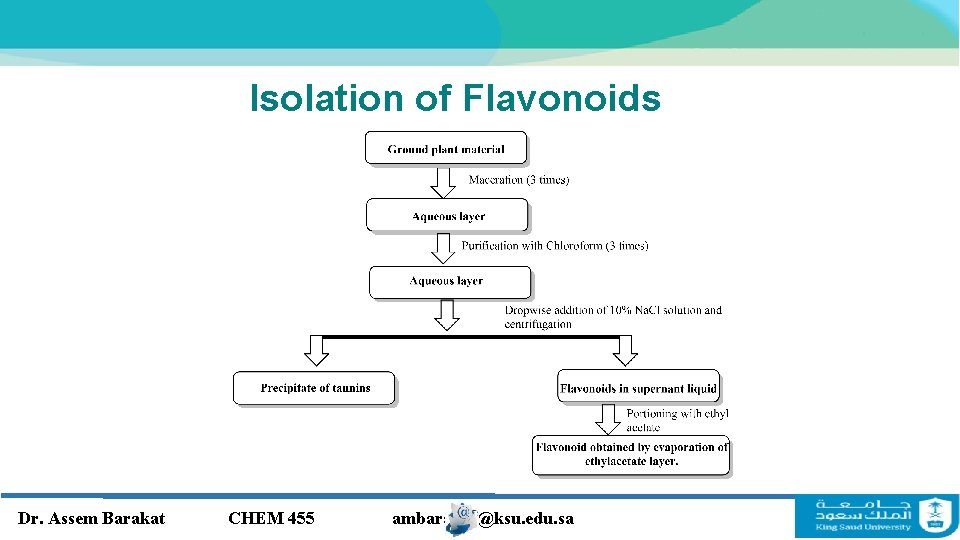
Isolation of Flavonoids Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

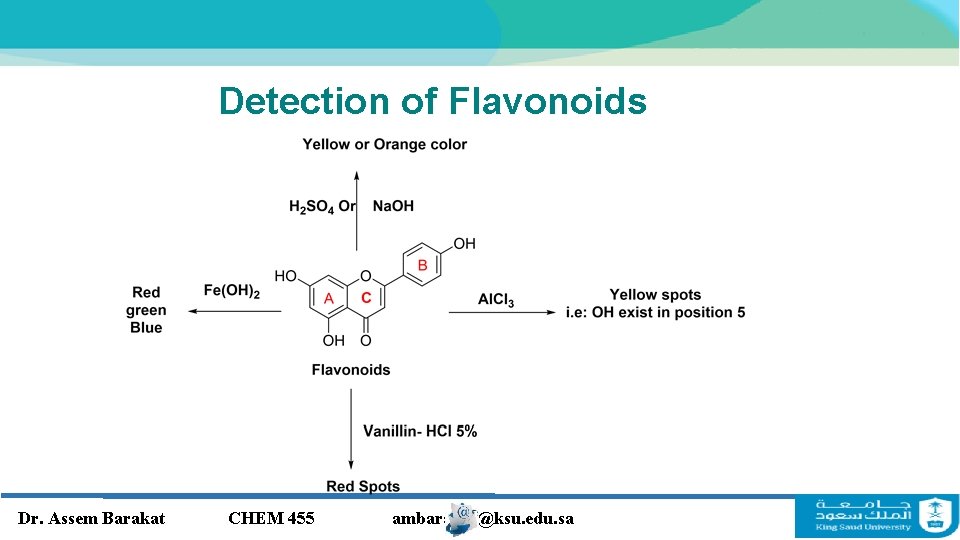
Detection of Flavonoids Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

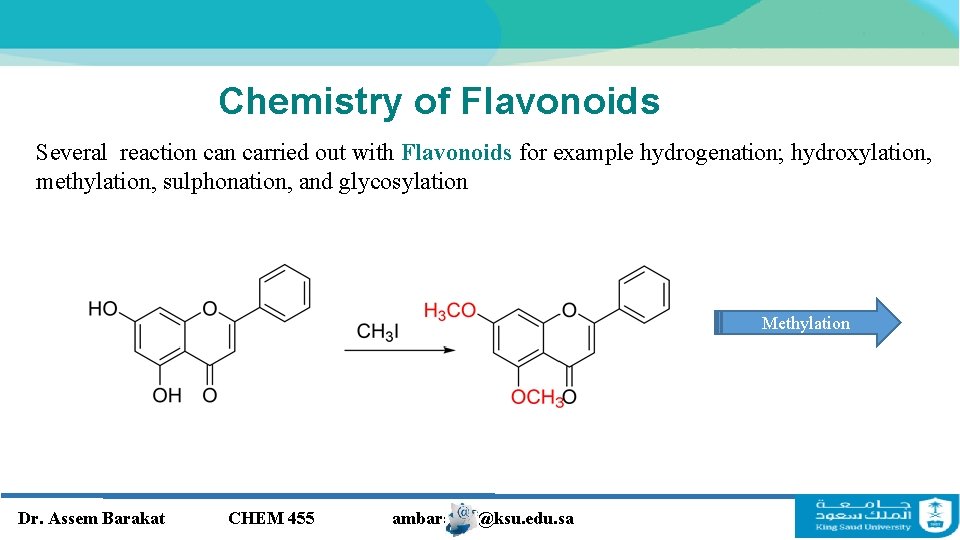
Chemistry of Flavonoids Several reaction carried out with Flavonoids for example hydrogenation; hydroxylation, methylation, sulphonation, and glycosylation Methylation Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

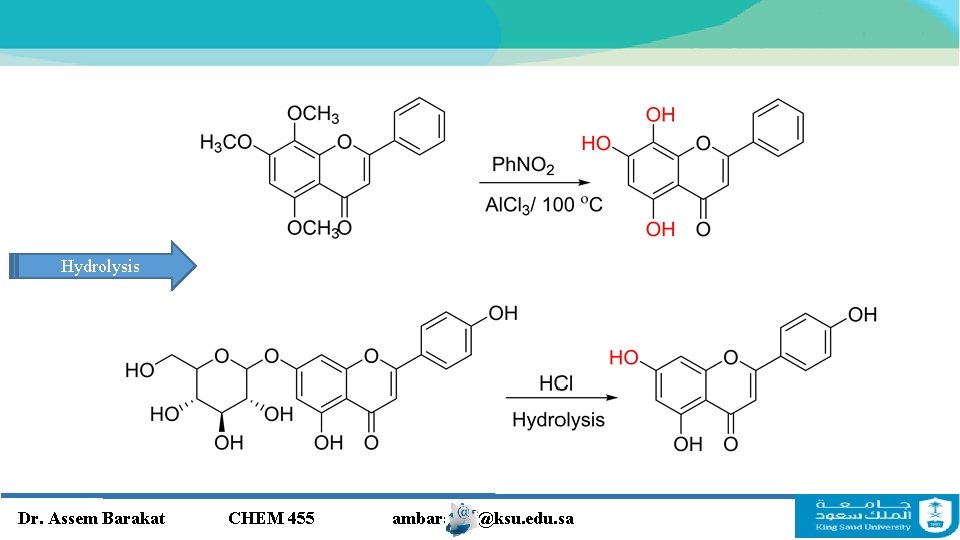
Hydrolysis Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

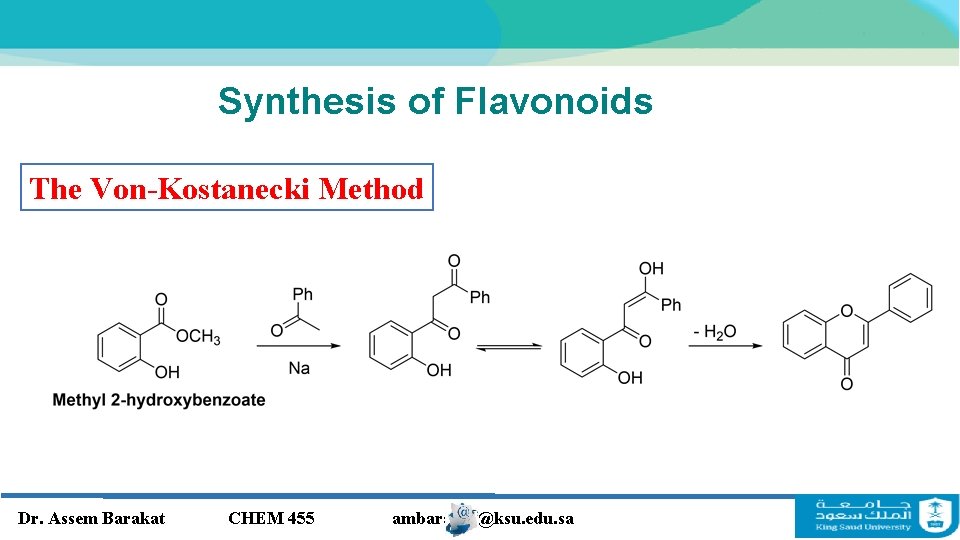
Synthesis of Flavonoids The Von-Kostanecki Method Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

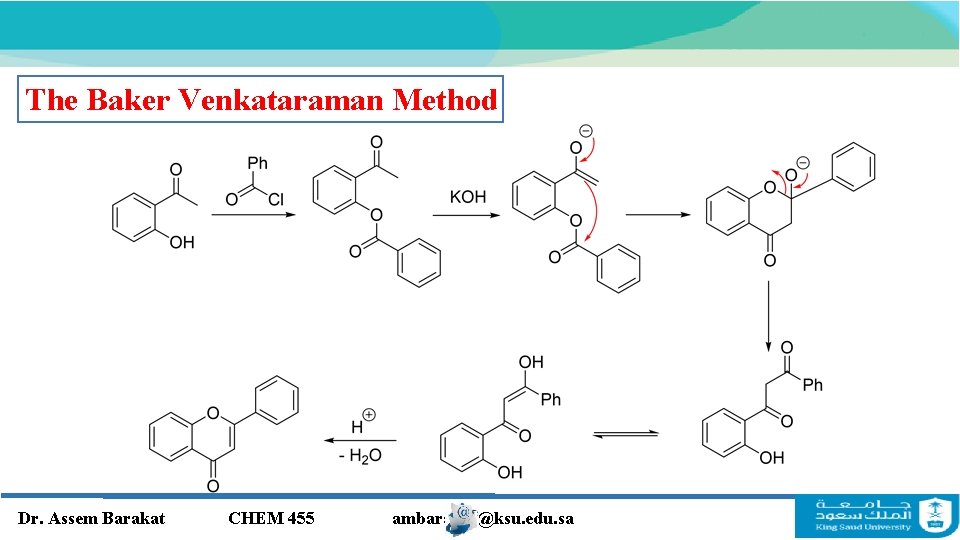
The Baker Venkataraman Method Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

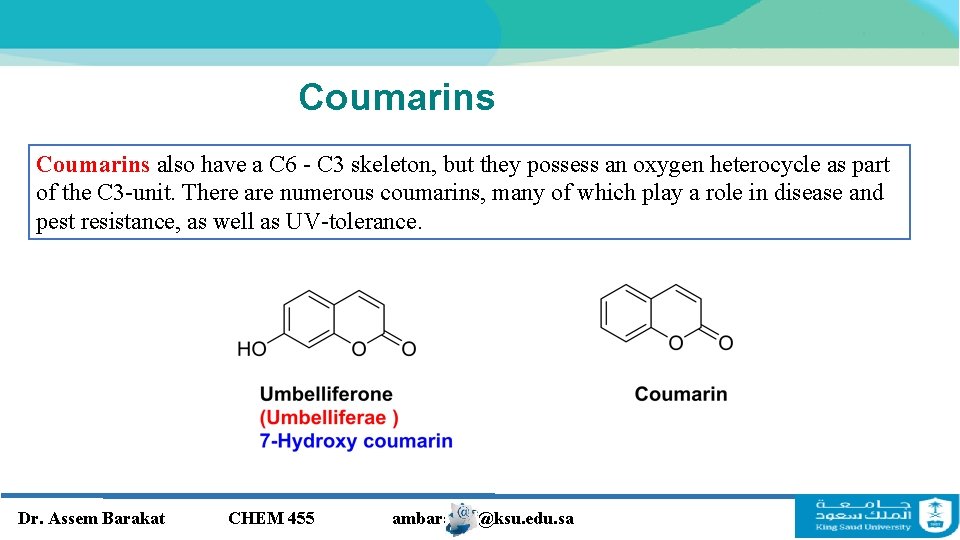
Coumarins also have a C 6 - C 3 skeleton, but they possess an oxygen heterocycle as part of the C 3 -unit. There are numerous coumarins, many of which play a role in disease and pest resistance, as well as UV-tolerance. Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

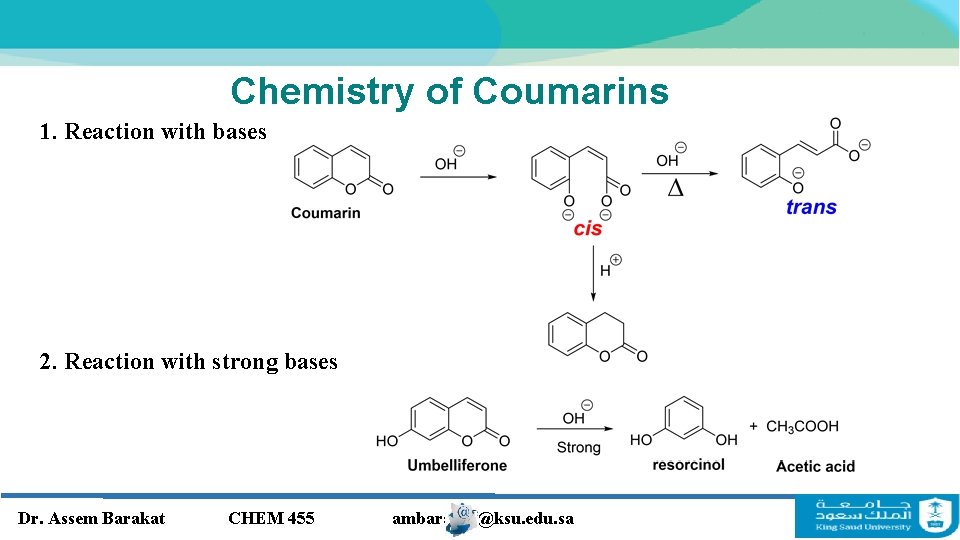
Chemistry of Coumarins 1. Reaction with bases 2. Reaction with strong bases Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

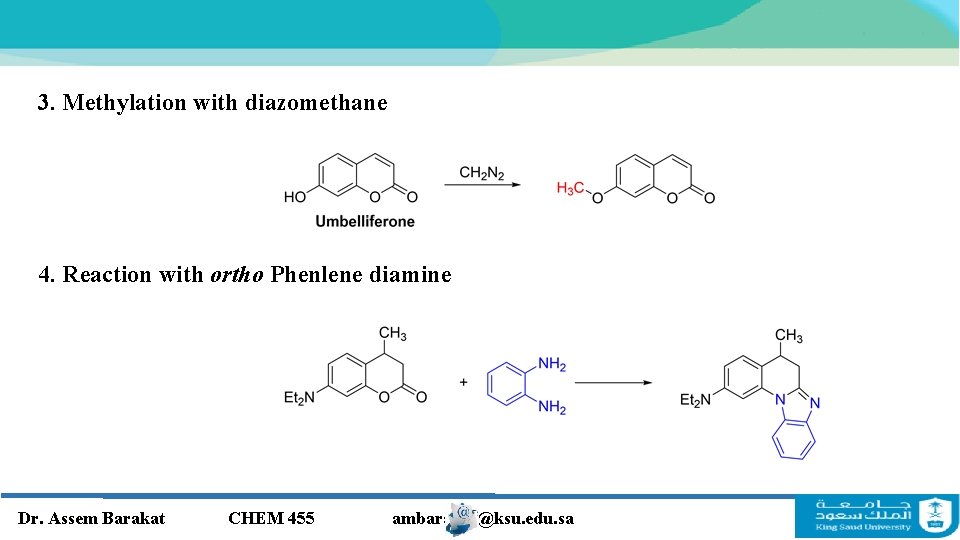
3. Methylation with diazomethane 4. Reaction with ortho Phenlene diamine Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

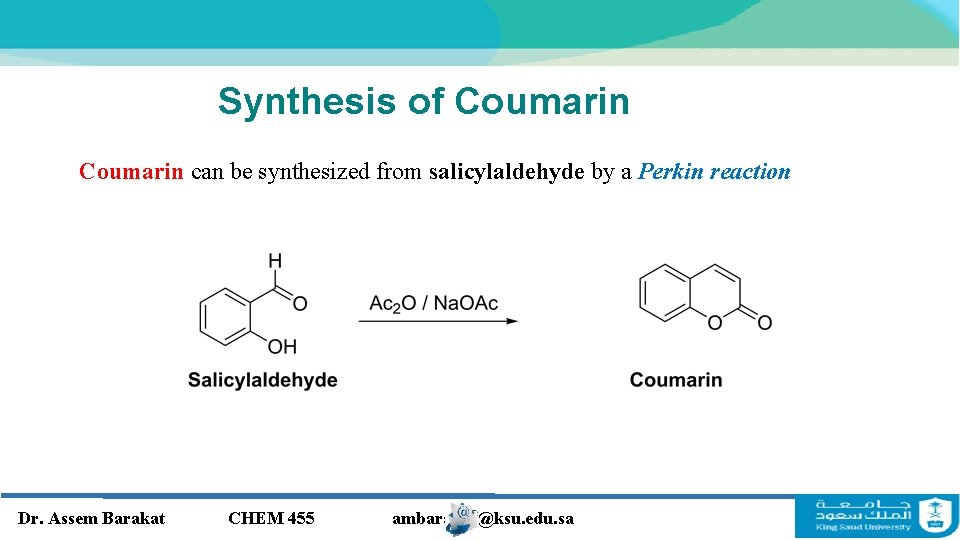
Synthesis of Coumarin can be synthesized from salicylaldehyde by a Perkin reaction Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

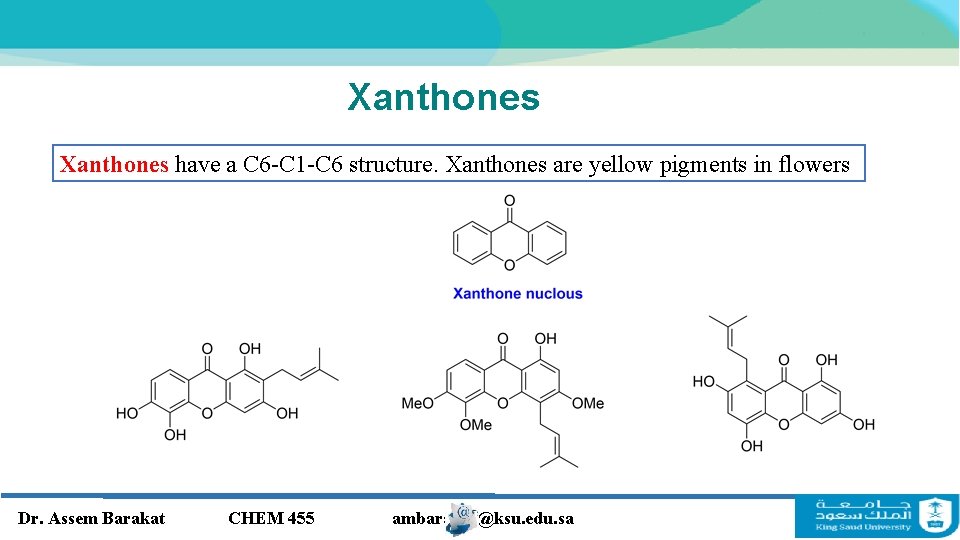
Xanthones have a C 6 -C 1 -C 6 structure. Xanthones are yellow pigments in flowers Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

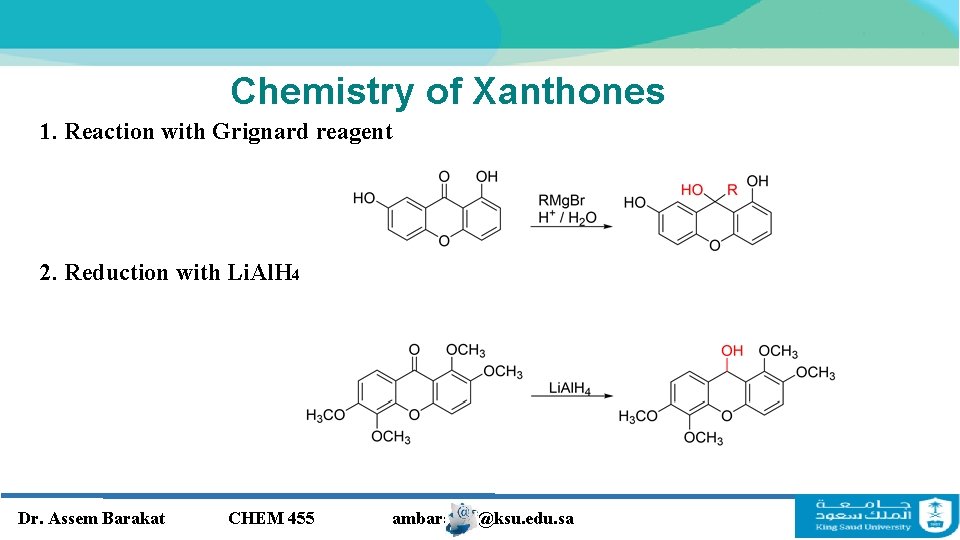
Chemistry of Xanthones 1. Reaction with Grignard reagent 2. Reduction with Li. Al. H 4 Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

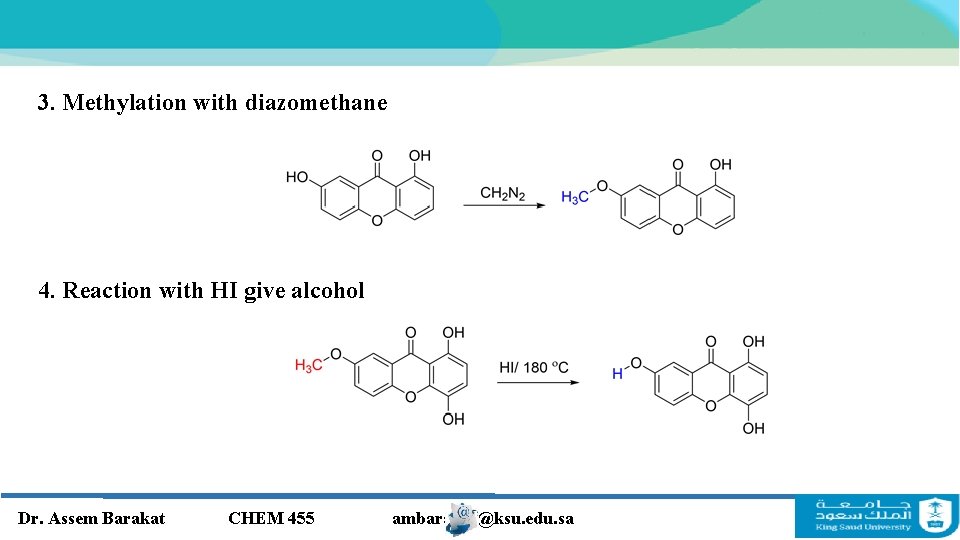
3. Methylation with diazomethane 4. Reaction with HI give alcohol Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

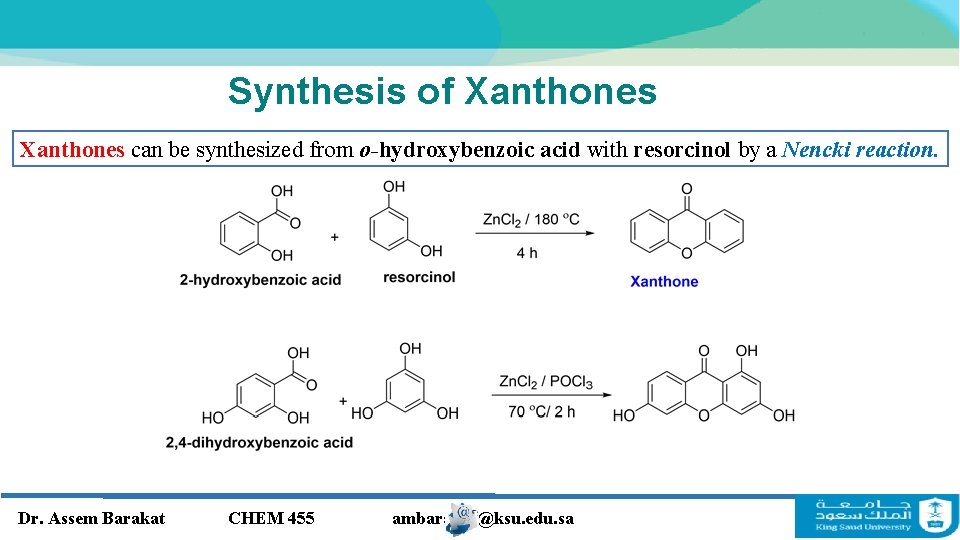
Synthesis of Xanthones can be synthesized from o-hydroxybenzoic acid with resorcinol by a Nencki reaction. Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa

Thank You and GOOD LUCK Dr. Assem Barakat CHEM 455 ambarakat@ksu. edu. sa