Chemistry of Living Things Part 2 Organic Inorganic






- Slides: 14

Chemistry of Living Things

Part 2 Organic & Inorganic Compounds: all living things are made up of both organic and inorganic substances. A. Inorganic Compounds: do NOT contain Carbon and Hydrogen. examples…water (H 20), salt (Na. Cl) B. Organic Compounds: contain both Carbon and Hydrogen examples…carbohydrates, proteins, lipids and nucleic acids

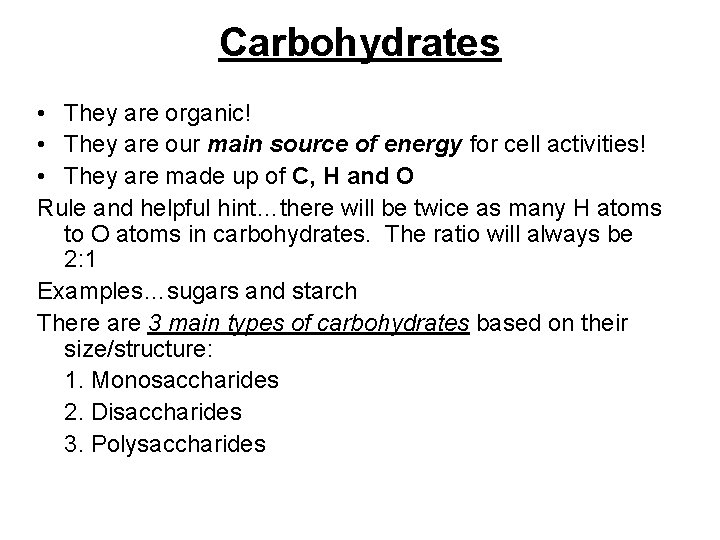
Carbohydrates • They are organic! • They are our main source of energy for cell activities! • They are made up of C, H and O Rule and helpful hint…there will be twice as many H atoms to O atoms in carbohydrates. The ratio will always be 2: 1 Examples…sugars and starch There are 3 main types of carbohydrates based on their size/structure: 1. Monosaccharides 2. Disaccharides 3. Polysaccharides

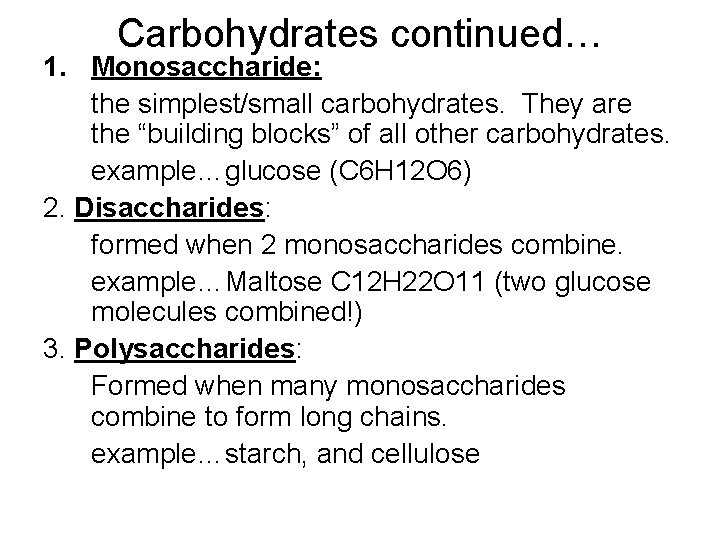
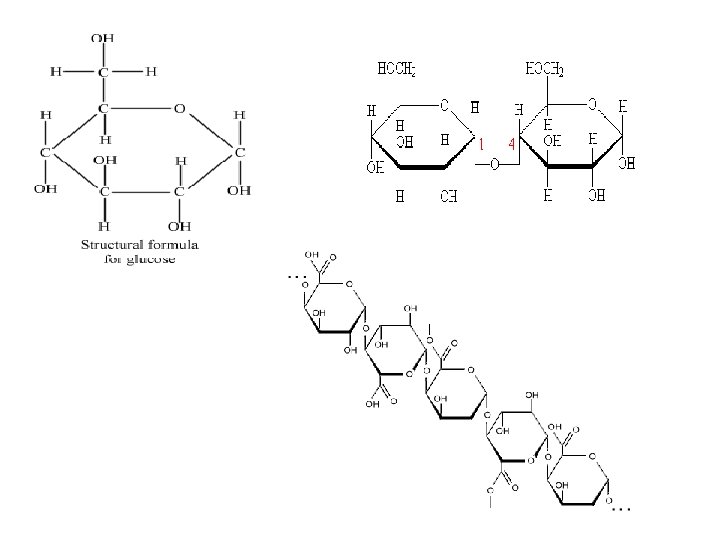
Carbohydrates continued… 1. Monosaccharide: the simplest/small carbohydrates. They are the “building blocks” of all other carbohydrates. example…glucose (C 6 H 12 O 6) 2. Disaccharides: formed when 2 monosaccharides combine. example…Maltose C 12 H 22 O 11 (two glucose molecules combined!) 3. Polysaccharides: Formed when many monosaccharides combine to form long chains. example…starch, and cellulose


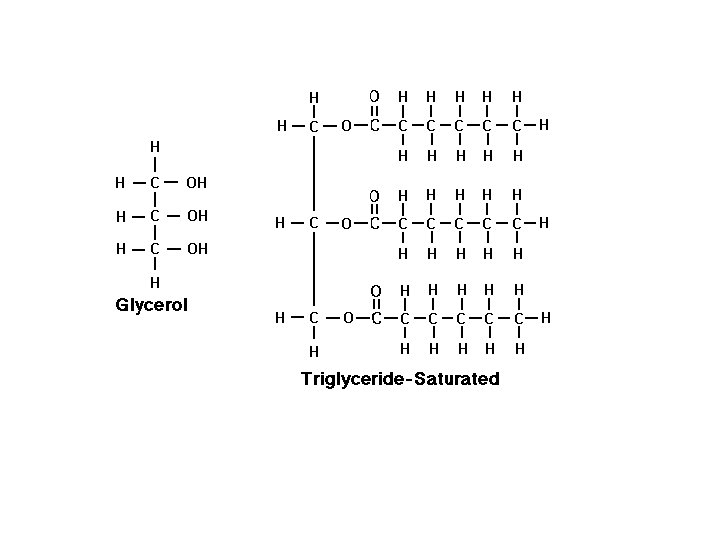
• • 1. 2. • • Lipids They are Organic! Lipids are fats and oils fats: solid at room temperature oils: liquid at room temperature Lipids contain the elements C, H and O (ratio of Hydrogen to Oxygen atoms is greater than 2: 1) The “building blocks” of lipids are fatty acids & glycerol Lipids are found in the cell membrane Extra food that isn’t used immediately for energy is changed to fat and stored… *lipids are a source of stored energy!!*


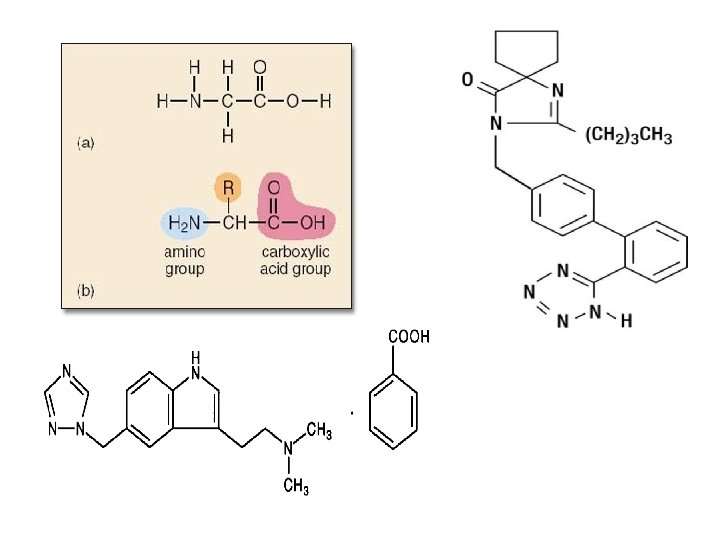
Proteins • • They are Organic! Made up of C, H, O and N The “building blocks” for proteins are amino acids. Proteins are found in different structures of living things and form important cell products…examples… enzymes, hormones, antibodies, hemoglobin! • Proteins also help in growth and repair! • There are 20 different amino acids found in living things. We can naturally make 10 of these. The other 10 we must get from food… • The ones we get from food are called essential amino acids


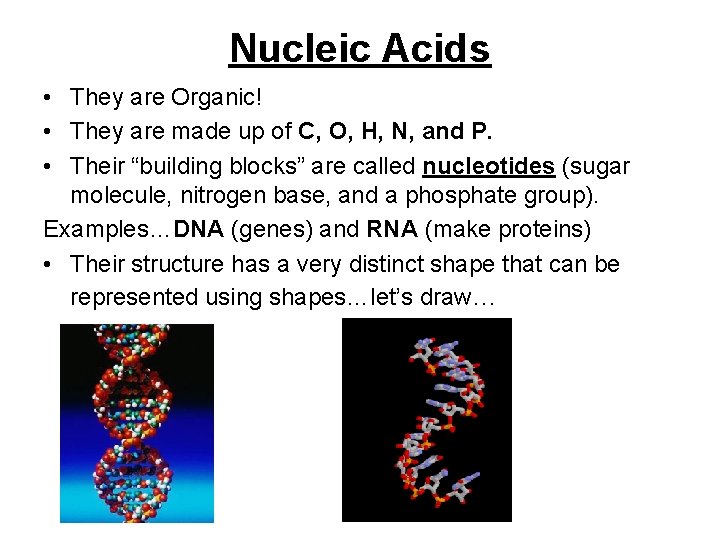
Nucleic Acids • They are Organic! • They are made up of C, O, H, N, and P. • Their “building blocks” are called nucleotides (sugar molecule, nitrogen base, and a phosphate group). Examples…DNA (genes) and RNA (make proteins) • Their structure has a very distinct shape that can be represented using shapes…let’s draw…

More on proteins…the example, Enzymes • Enzymes are a type of protein. • They control the rate of all chemical reactions that occur in living things. • They are considered a catalyst - catalyst: something that can either speed up or slow down a reaction. • They work with co-enzymes: substance that helps the enzyme function. example…vitamins! • Rule and helpful hint…all enzymes end in the letters – ”ase” * if an enzyme doesn’t function, metabolic activities (life functions) can’t occur, therefore it’s important to eat a well balanced diet and get your vitamins!

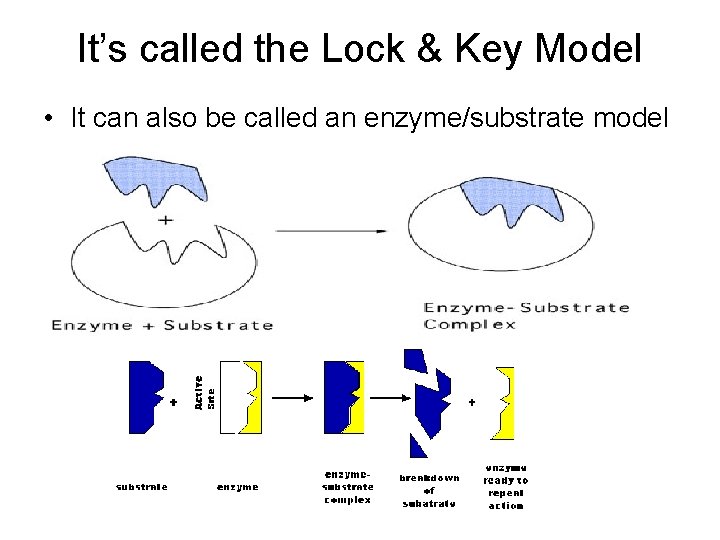
Enzymes continued… Factors that affect enzymes: 1. Temperature 2. Substrate concentration 3. p. H How Enzymes Work: The enzyme has an area on its surface with a very distinct shape called an active site. Only specific substances that can fit into the active site can bond with the enzyme and get it to work. Substrate: the substance that can bond with the enzyme. Active Site: the area on an enzyme where a substrate bonds.

It’s called the Lock & Key Model • It can also be called an enzyme/substrate model

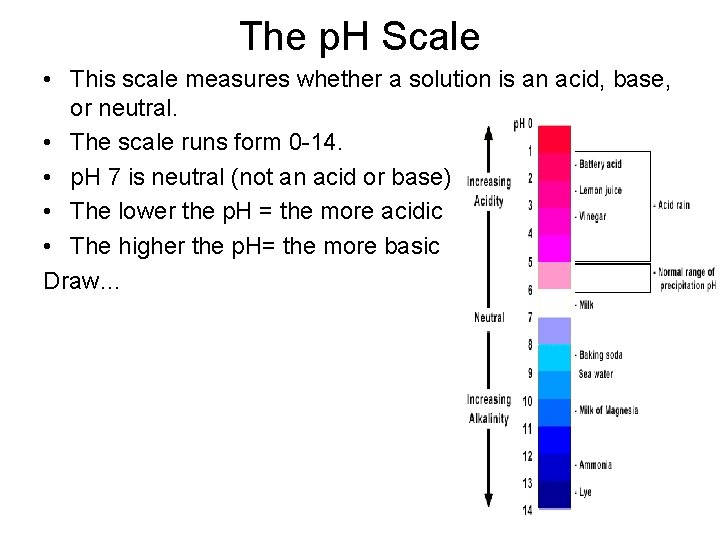
The p. H Scale • This scale measures whether a solution is an acid, base, or neutral. • The scale runs form 0 -14. • p. H 7 is neutral (not an acid or base) • The lower the p. H = the more acidic • The higher the p. H= the more basic Draw…