CHEMISTRY OF LIVING THINGS PART 1 BASIC CHEMISTRY
CHEMISTRY OF LIVING THINGS PART 1 BASIC CHEMISTRY

What is Chemistry? � Chemistry � Matter is the study of matter. is anything that has mass and takes up space.

What is an atom? � An atom is the smallest unit of matter. � An atom can NOT be divided any smaller
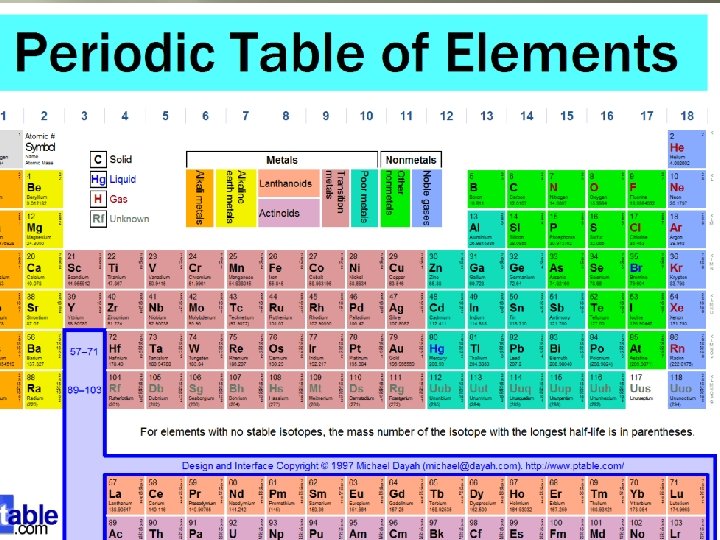
What is an element? � Any substance that is made up of one kind of atom is an element. � Examples of Elements: �Gold �Copper �Iron �Oxygen �Hydrogen All elements are presented by symbols. Oxygen – O Carbon – C Iron – Fe All known elements are on the Periodic Table. Do you know any other elements? _________________
What is an element?
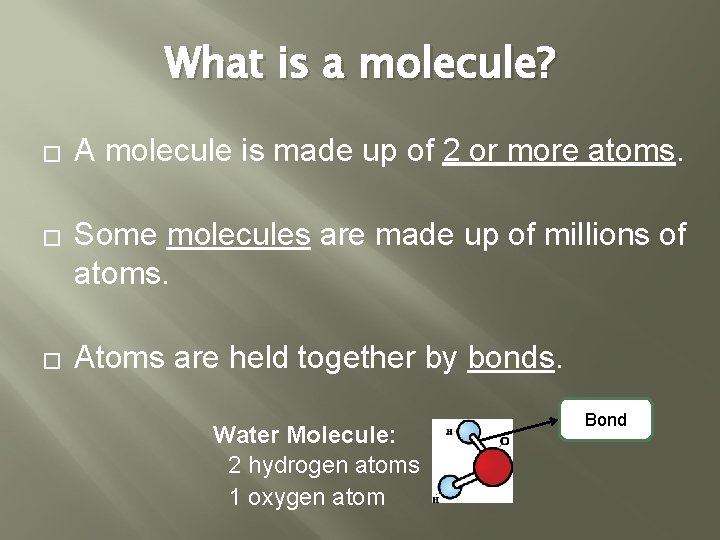
What is a molecule? � � � A molecule is made up of 2 or more atoms. Some molecules are made up of millions of atoms. Atoms are held together by bonds. Water Molecule: 2 hydrogen atoms 1 oxygen atom Bond
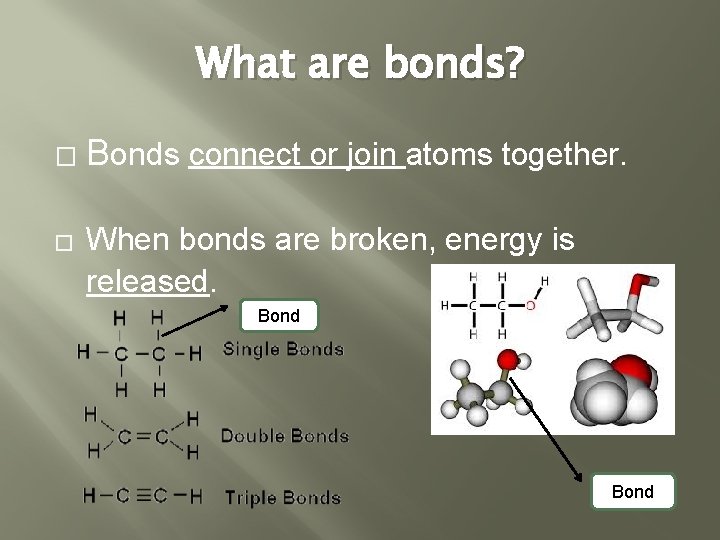
What are bonds? � � Bonds connect or join atoms together. When bonds are broken, energy is released. Bond

What are compounds? � A compound is a substance made of atoms. The elements in compounds lose their own properties. For example: �The elements hydrogen & oxygen are both gases. When 2 hydrogen atoms bond with 1 oxygen atom it forms a liquid. . . WATER! Water is a compound! H 2 O List as many other compounds as you can? _________________

What are Chemical Formulas? �A chemical formula is a short way of writing a compound. � Just like each element has its own chemical symbol, each compound has its own formula.

What does a chemical formula included? �A 1. 2. chemical formula tell us: The elements in the compound How many atoms of each element For example: The chemical formula for carbon dioxide Co 2 1 carbon atom and 2 oxygen atoms.

What is a chemical equation? � A chemical equation shows the starting reactants and the end products of a chemical reaction. � Reactants – The “ingredients” needed for a chemical reaction. � Products – What is created after the chemical reaction.
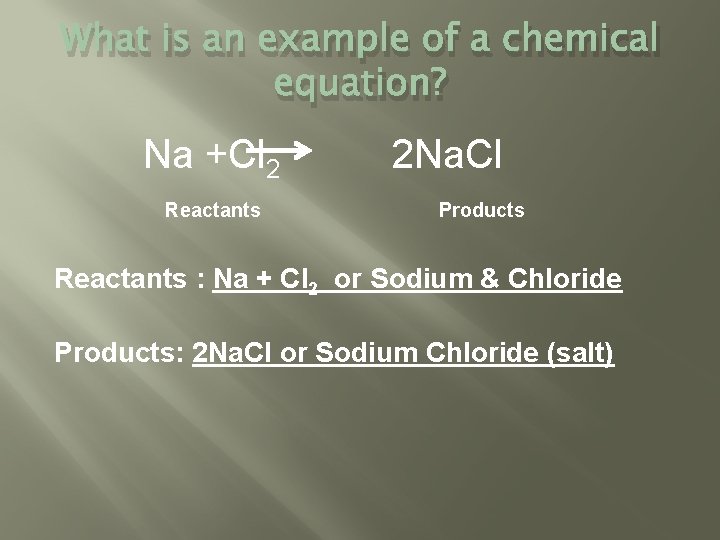
What is an example of a chemical equation? Na +Cl 2 Reactants 2 Na. Cl Products Reactants : Na + Cl 2 or Sodium & Chloride Products: 2 Na. Cl or Sodium Chloride (salt)
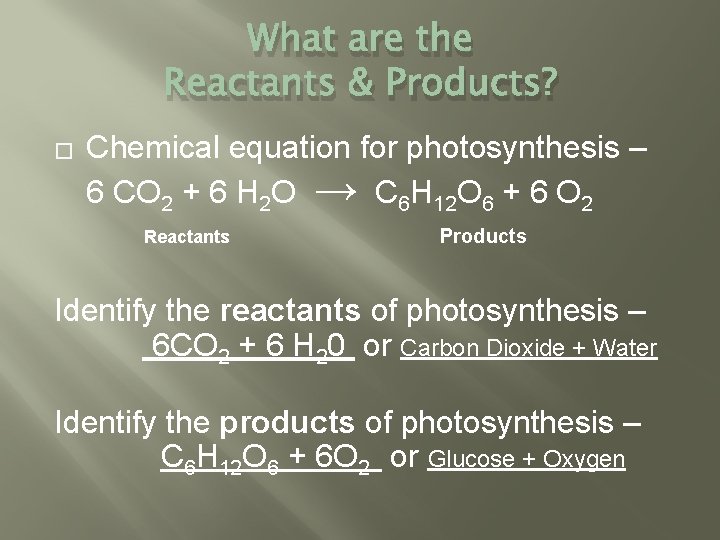
What are the Reactants & Products? � Chemical equation for photosynthesis – 6 CO 2 + 6 H 2 O → C 6 H 12 O 6 + 6 O 2 Reactants Products Identify the reactants of photosynthesis – 6 CO 2 + 6 H 20 or Carbon Dioxide + Water Identify the products of photosynthesis – C 6 H 12 O 6 + 6 O 2 or Glucose + Oxygen

What does chemistry have to do with living things? � Let’s watch the Brain. POP to find out! Click the link below � Brainpop: Body Chemistry
- Slides: 14