Chemistry of Life What is organic chemistry What







- Slides: 30

Chemistry of Life What is organic chemistry? What are carbohydrates? What are lipids? What are proteins? What are nucleic acids? Learning Targets: I can 1. Name the four major organic molecules. 2. Explain the following for each of the organic molecules: Øthe chemical elements that make up the molecule Øthe building blocks/structure Øtheir functions Øgive some examples

• Go to my Living Environment webpage and watch the Biomolecules video- Amoeba sisters (and for your review you can complete video recap WS) •

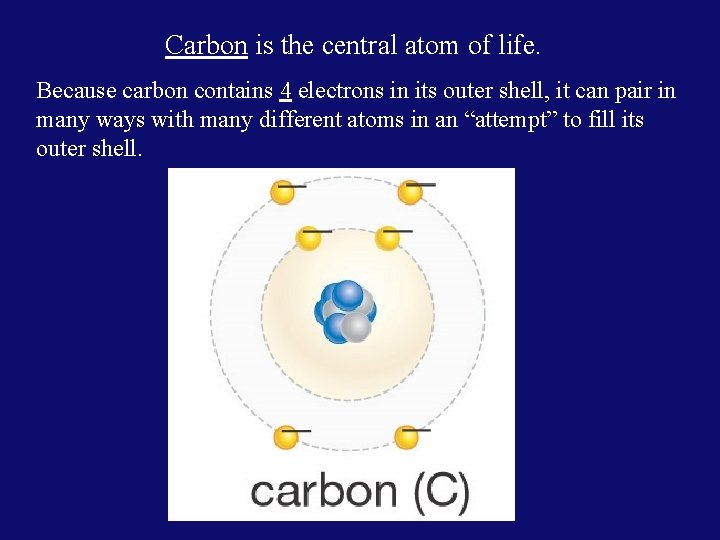
Carbon is the central atom of life. Because carbon contains 4 electrons in its outer shell, it can pair in many ways with many different atoms in an “attempt” to fill its outer shell.

Organic Chemistry • ORGANIC means comes from and found in LIVING things • Organic compounds must contain both Carbon (C) and Hydrogen (H) • Inorganic – Doesn’t contain both C and H • Inorganic substances that living things rely on: • Water (H 2 O), Salt (Na. Cl), Hydrochloric acid (HCl) Practice: Organic or Inorganic? ? ? • • • H 2 O = ______ (water) Na. Cl = ______ (salt) C 6 H 12 O 6 = ______ (sugar/glucose) CH 4 = _______ (methane) CO 2 = _______ (carbon dioxide) O 2 = _______ (oxygen)

Macromolecules • Means “Giant molecules” • Small things (MONOMERS) join together to make large things (POLYMERS)…this is called Polymerization

Making and Breaking polymers 1. Dehydration synthesis: (condensation) • combining simple molecules to form a more complex one with the removal of water • Example: – monomer + monomer polymer + water 2. Hydrolysis (digestion): • adding a water molecule to a (polymer) to split it into small monomers • Example: – polymer + water monomer + monomer

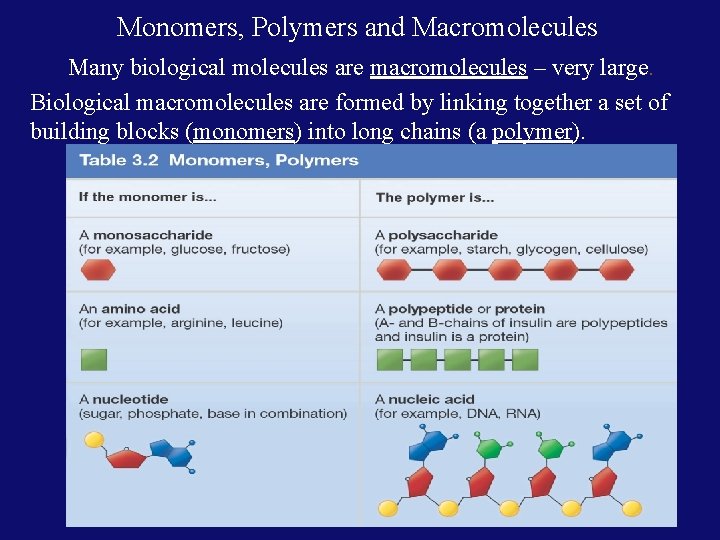
Monomers, Polymers and Macromolecules Many biological molecules are macromolecules – very large. Biological macromolecules are formed by linking together a set of building blocks (monomers) into long chains (a polymer).

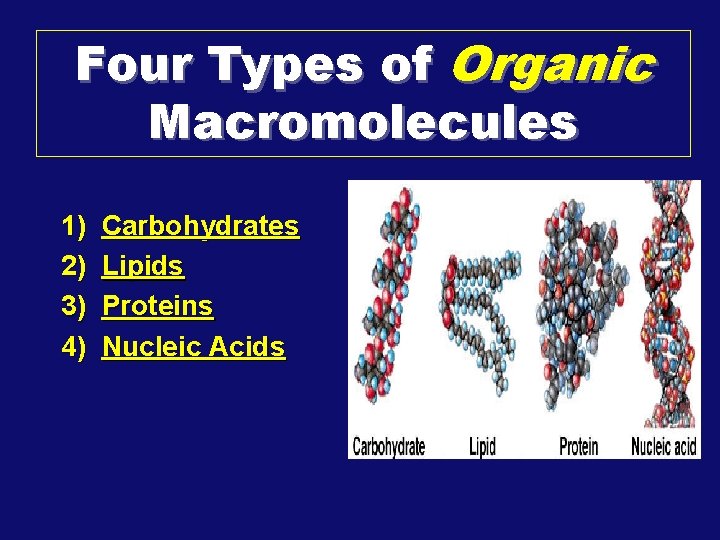
Four Types of Organic Macromolecules 1) 2) 3) 4) Carbohydrates Lipids Proteins Nucleic Acids

Carbs: The Video Clip

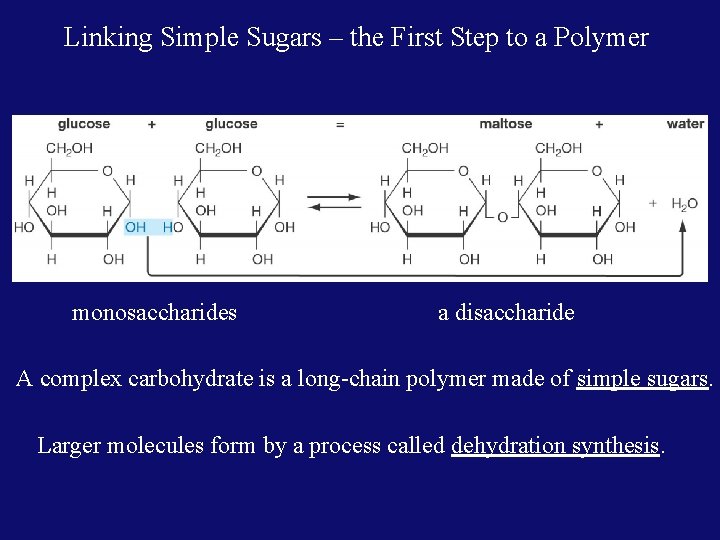
Linking Simple Sugars – the First Step to a Polymer monosaccharides a disaccharide A complex carbohydrate is a long-chain polymer made of simple sugars. Larger molecules form by a process called dehydration synthesis.

1. Carbohydrates (SUGARS)- aka “saccharides” a. Elements Present: • Carbon, Hydrogen, Oxygen • 1: 2: 1 ratio ex. C 6 H 12 O 6 b. Job (Function) in Living Things: • Main source of FOOD ENERGY c. Building Blocks: • Called Simple sugars • End in –ose ex. glucose • Linked together to make complex (BIG) sugars • Glucose is a simple sugar • Many glucose molecules linked together makes STARCH • STARCH is a complex (BIG) sugar

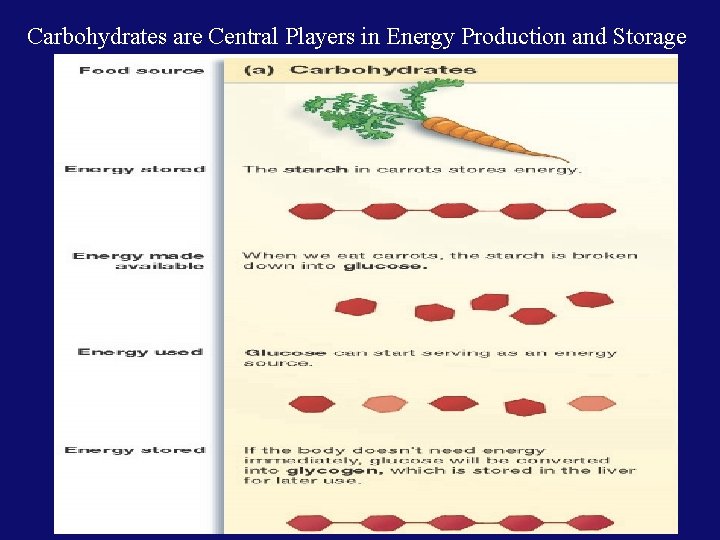
Carbohydrates are Central Players in Energy Production and Storage

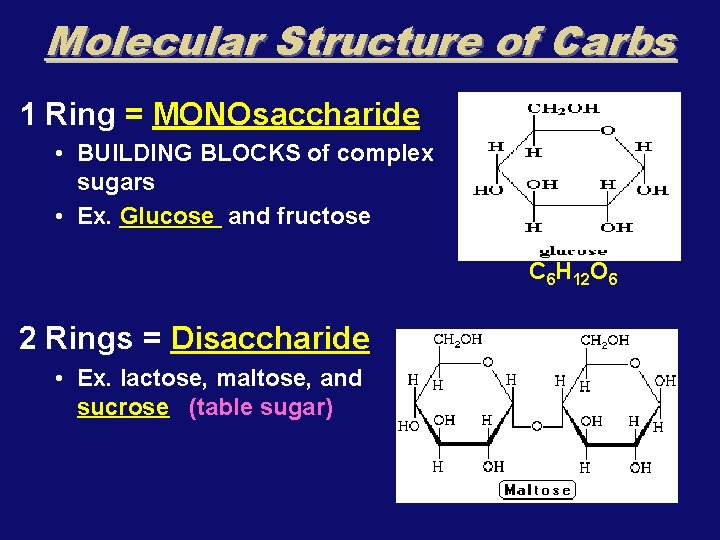
Molecular Structure of Carbs 1 Ring = MONOsaccharide • BUILDING BLOCKS of complex sugars • Ex. Glucose and fructose C 6 H 12 O 6 2 Rings = Disaccharide • Ex. lactose, maltose, and sucrose (table sugar)

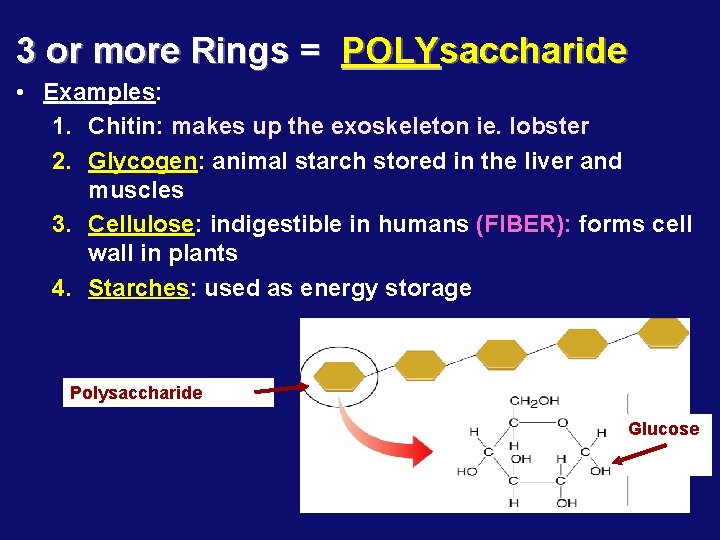
3 or more Rings = POLYsaccharide • Examples: 1. Chitin: makes up the exoskeleton ie. lobster 2. Glycogen: animal starch stored in the liver and muscles 3. Cellulose: indigestible in humans (FIBER): forms cell wall in plants 4. Starches: used as energy storage Polysaccharide Glucose

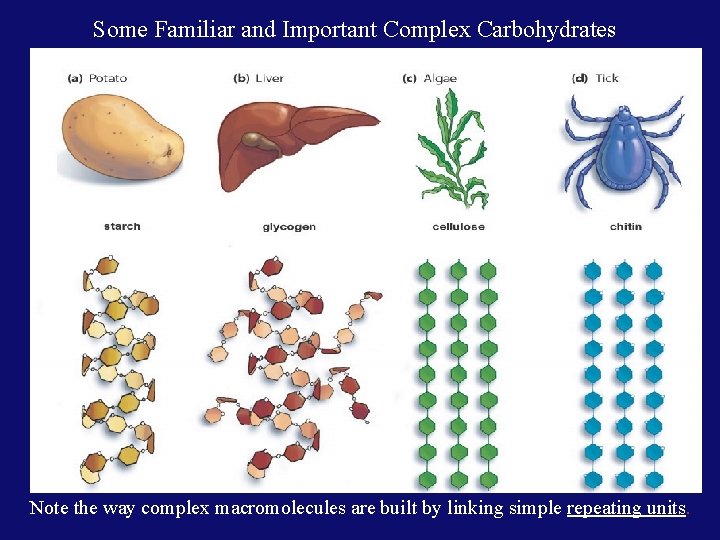
Some Familiar and Important Complex Carbohydrates Note the way complex macromolecules are built by linking simple repeating units.

Lipids: The Video Clip

2. Lipids (Fats) a. Elements: • Carbon, Hydrogen, Oxygen • Mostly H and O (H and O not in a 2: 1 ratio) b. Functions (Jobs) in Living Things: • Stores energy and insulates • Parts of cell membrane structure • Chemical messengers (hormones)

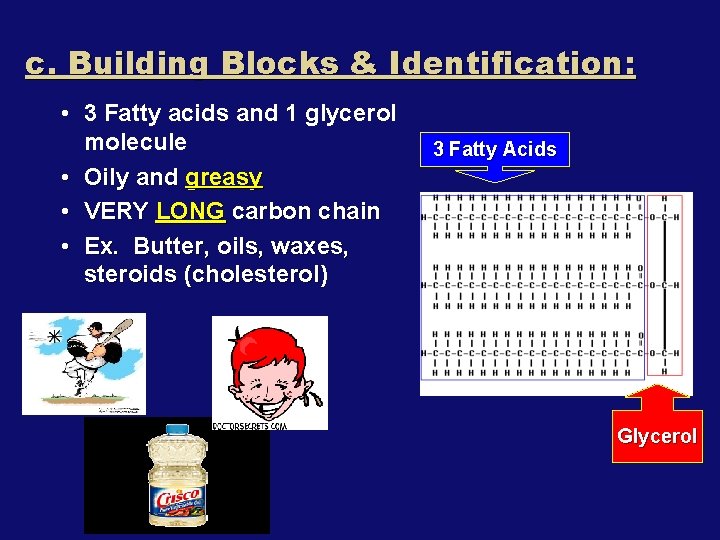
c. Building Blocks & Identification: • 3 Fatty acids and 1 glycerol molecule • Oily and greasy • VERY LONG carbon chain • Ex. Butter, oils, waxes, steroids (cholesterol) 3 Fatty Acids Glycerol

Protein: The Video Clip

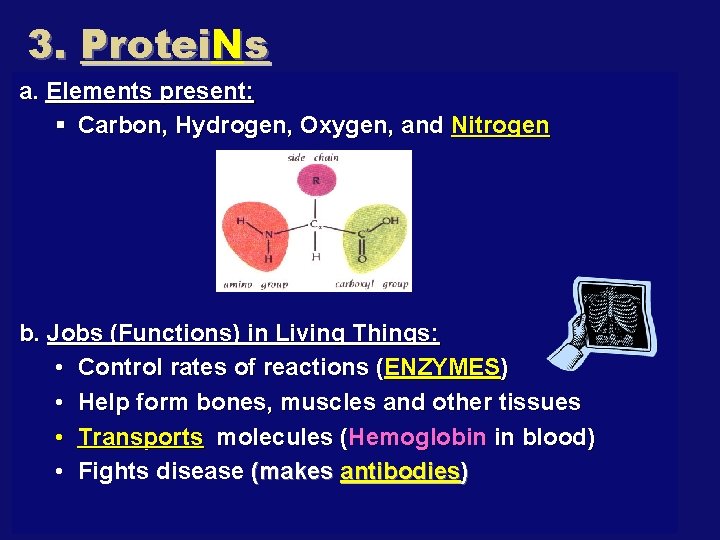
3. Protei. Ns a. Elements present: § Carbon, Hydrogen, Oxygen, and Nitrogen b. Jobs (Functions) in Living Things: • Control rates of reactions (ENZYMES) • Help form bones, muscles and other tissues • Transports molecules (Hemoglobin in blood) • Fights disease (makes antibodies)

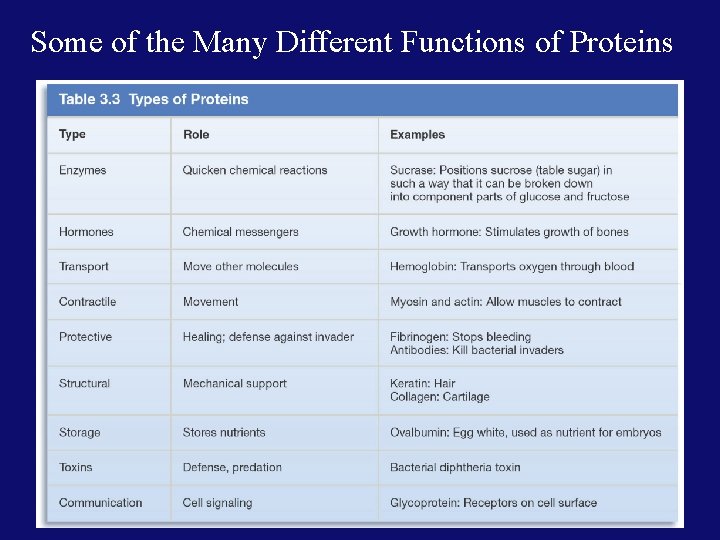
Some of the Many Different Functions of Proteins

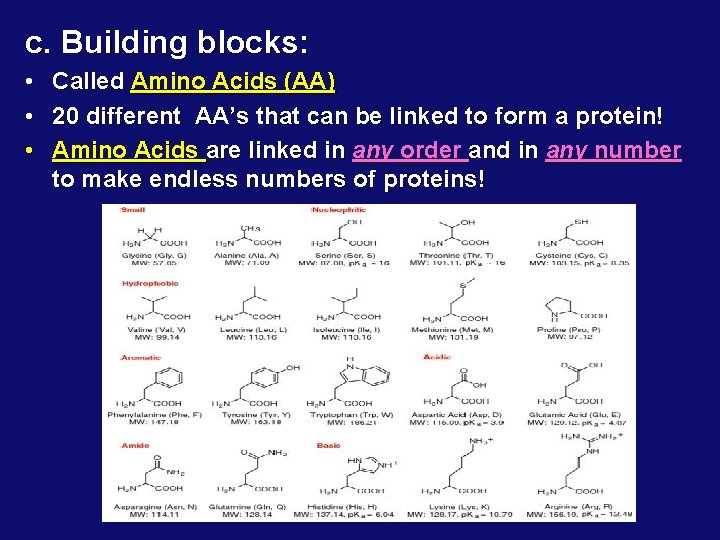
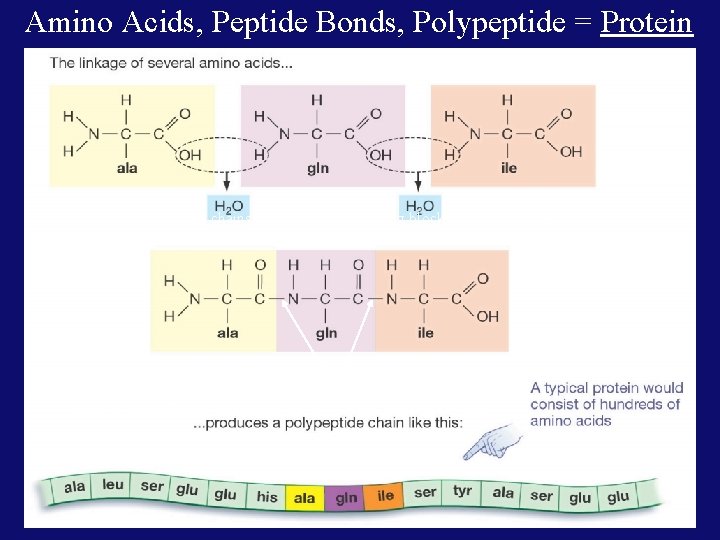
c. Building blocks: • • • Called Amino Acids (AA) 20 different AA’s that can be linked to form a protein! Amino Acids are linked in any order and in any number to make endless numbers of proteins!

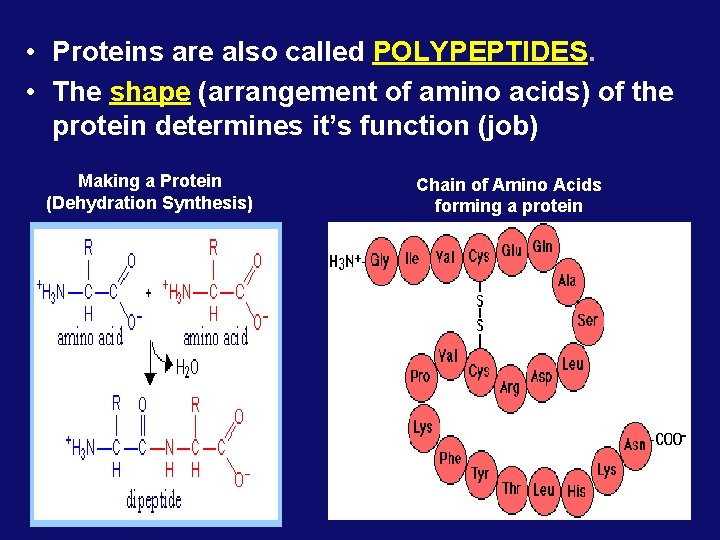
• Proteins are also called POLYPEPTIDES. • The shape (arrangement of amino acids) of the protein determines it’s function (job) Making a Protein (Dehydration Synthesis) Chain of Amino Acids forming a protein

Amino Acids, Peptide Bonds, Polypeptide = Proteins are linear chains of 20 different building blocks called amino acids. Peptide bonds Amino acids are linked by peptide bonds – a form of covalent bond.

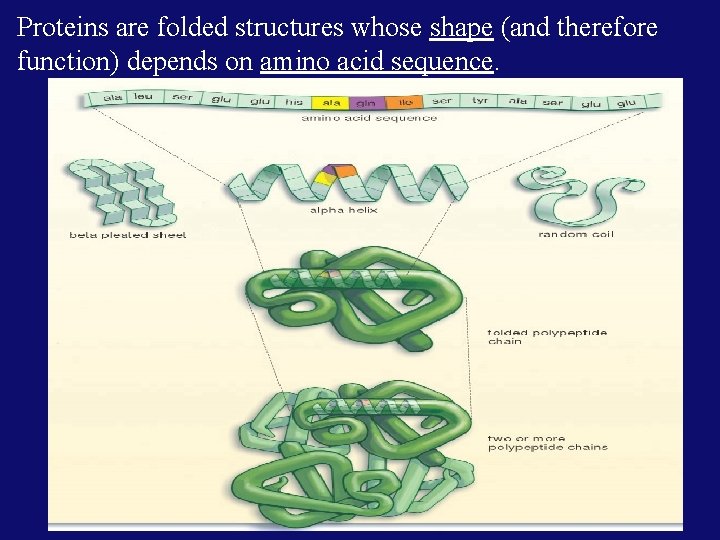
Proteins are folded structures whose shape (and therefore function) depends on amino acid sequence.

Nucleic Acids: The Video Clip

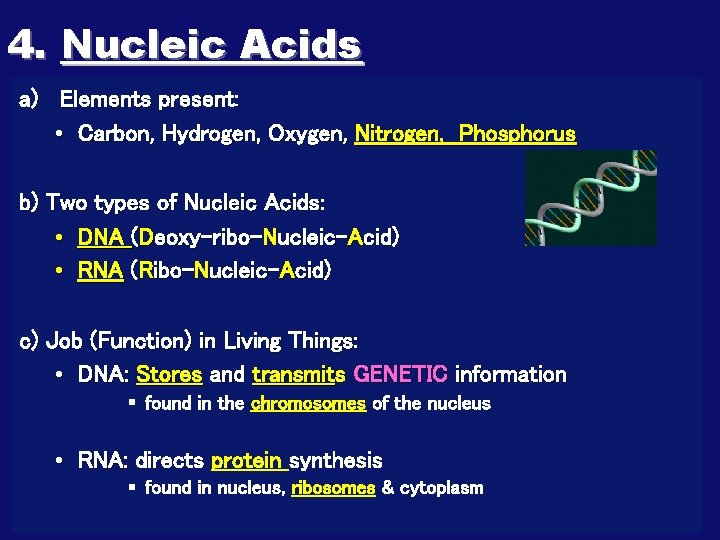
4. Nucleic Acids a) Elements present: • Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus b) Two types of Nucleic Acids: • DNA (Deoxy-ribo-Nucleic-Acid) • RNA (Ribo-Nucleic-Acid) c) Job (Function) in Living Things: • DNA: Stores and transmits GENETIC information § found in the chromosomes of the nucleus • RNA: directs protein synthesis § found in nucleus, ribosomes & cytoplasm

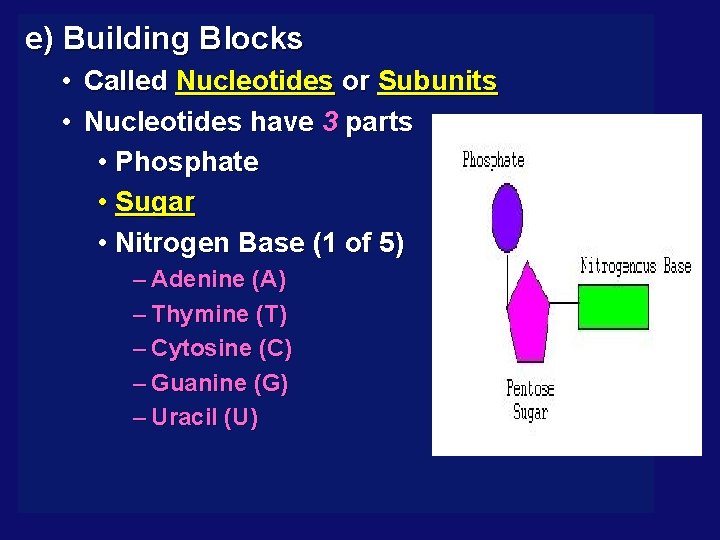
e) Building Blocks • Called Nucleotides or Subunits • Nucleotides have 3 parts • Phosphate • Sugar • Nitrogen Base (1 of 5) – Adenine (A) – Thymine (T) – Cytosine (C) – Guanine (G) – Uracil (U)

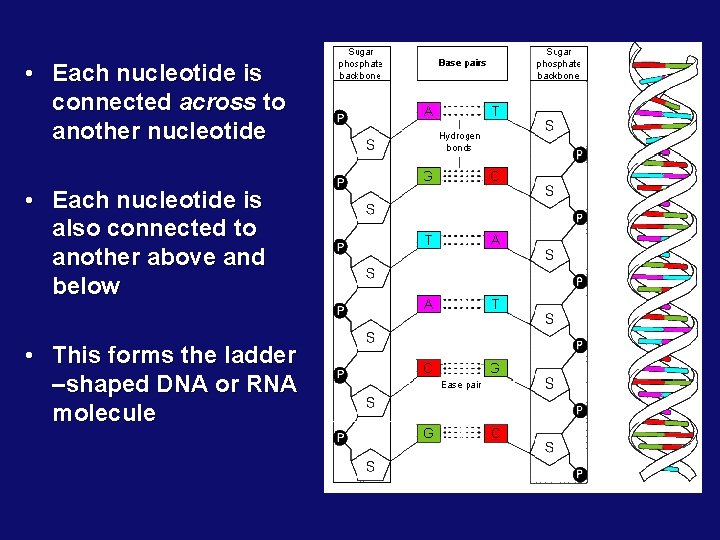
• Each nucleotide is connected across to another nucleotide • Each nucleotide is also connected to another above and below • This forms the ladder –shaped DNA or RNA molecule

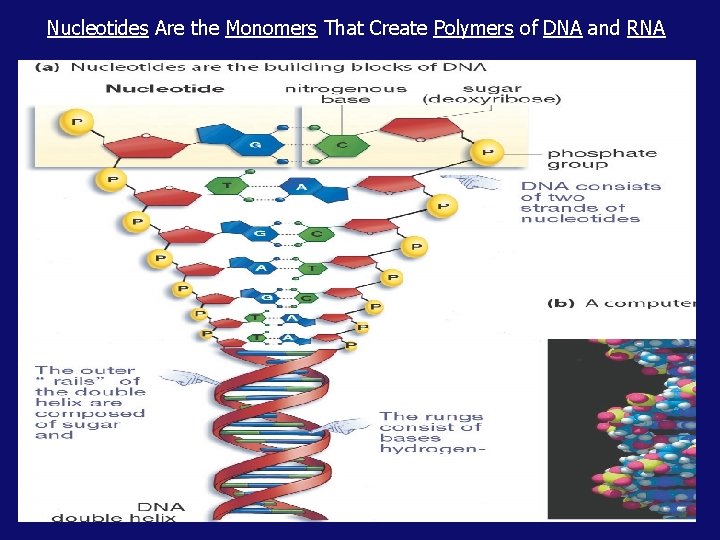
Nucleotides Are the Monomers That Create Polymers of DNA and RNA