Chemistry of Life Section 1 Atoms Every living




























- Slides: 42

Chemistry of Life Section 1 Atoms • Every living and nonliving thing is made of matter. Matter is anything that has mass and takes up space. • All matter is made of very small particles called atoms. An atom is the smallest unit of matter that cannot be broken down by chemical means. • An atom has a positively charged core surrounded by a negatively charged region.

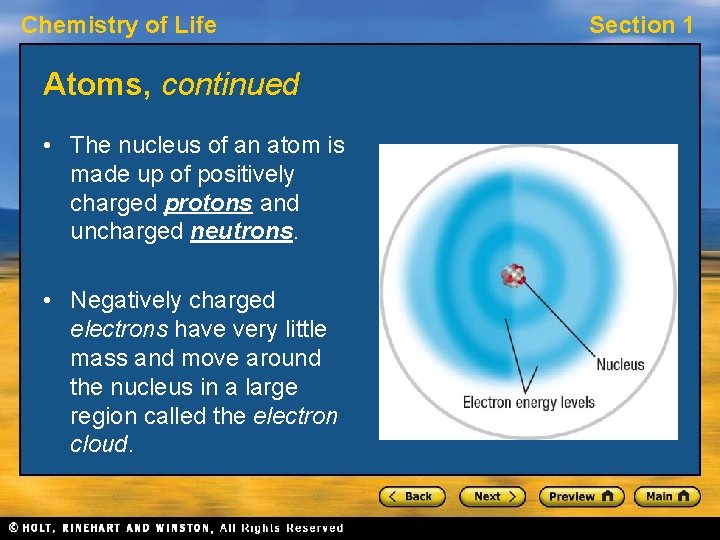
Chemistry of Life Atoms, continued • The nucleus of an atom is made up of positively charged protons and uncharged neutrons. • Negatively charged electrons have very little mass and move around the nucleus in a large region called the electron cloud. Section 1

Chemistry of Life Section 1 Atoms, continued Elements • An element is a substance made up of atoms that have the same number of protons. • For example, each atom of the element carbon has six protons. • Atoms of an element may have different numbers of neutrons. These atoms are called isotopes of elements.

Chemistry of Life Section 1 Chemical Bonds • The electron cloud of an atom may have levels. • Electrons in the outermost level, or shell, are called valence electrons. • Atoms tend to combine with each other such that eight electrons will be in the valence shell. • When atoms combine, a force called a chemical bond holds them together.

Chemistry of Life Section 1 Chemical Bonds, continued • Chemical bonds form between groups of atoms because most atoms become stable when they have eight electrons in the valence shell. • When atoms of different elements combine, a compound forms. – A compound is a substance made of the bonded atoms of two or more elements.

Chemistry of Life Section 1 Chemical Bonds, continued Covalent Bonding • One way that atoms bond is by sharing valence electrons to form a covalent bond. • A molecule is a group of atoms held together by covalent bonds. • A water molecule, H 2 O, forms when an oxygen atom forms covalent bonds with two hydrogen atoms.

Chemistry of Life Visual Concept: Covalent Bonding Section 1

Chemistry of Life Section 1 Chemical Bonds, continued Ionic Bonding • Atoms can achieve a stable valence level by losing or gaining electrons, resulting in a positive or negative charge. • An ion is an atom or group of atoms that has an electric charge because it has gained or lost electrons. • The attractive force between oppositely charged ions is an ionic bond.

Chemistry of Life Section 1 Ionic Bonding in Salt Click to animate the image.

Chemistry of Life Section 1 Polarity • In some covalent bonds, the shared electrons are attracted more strongly to one atom than to the other. • As a result, one end of the molecule has a partial negative charge, while the opposite end has a partial positive charge. • Molecules with partial charges on opposite ends are said to be polar.

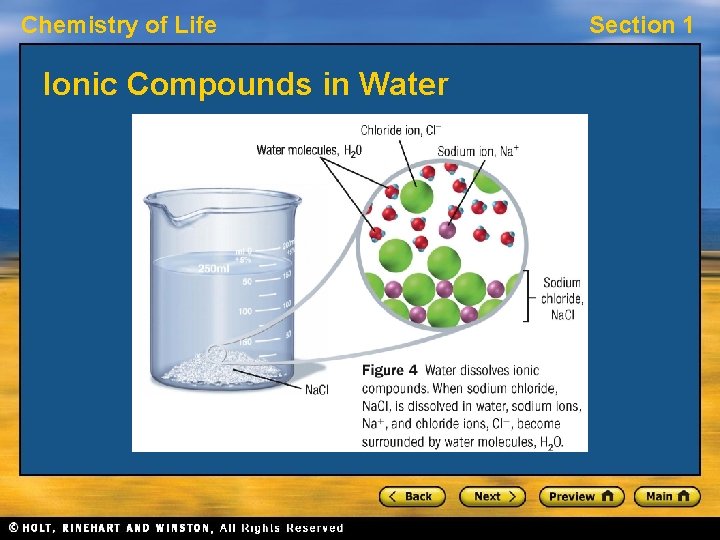
Chemistry of Life Section 1 Polarity, continued Solubility • The partially charged ends of polar molecules attract opposite charges. • Because of this behavior, polar molecules can dissolve other polar molecules and ionic compounds. For example, water can dissolve sugar and salt. • Nonpolar substances, such as oil, grease, and wax, do not dissolve well in water.

Chemistry of Life Ionic Compounds in Water Section 1

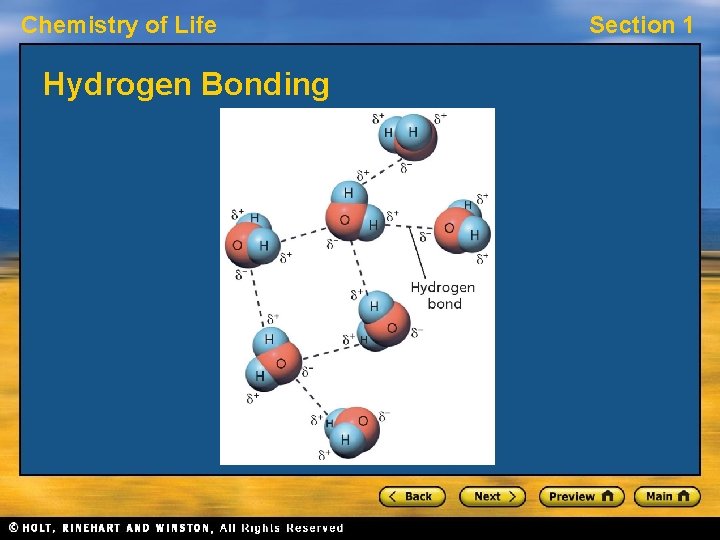
Chemistry of Life Section 1 Polarity, continued Hydrogen Bonds • When bonded to an oxygen, nitrogen, or fluorine atom (FON), a hydrogen atom has a partial charge nearly as great as a proton’s charge. • It attracts the negative pole of other nearby molecules. This attraction, called a hydrogen bond, is stronger than attractions between other molecules, but not as strong as covalent bonds. • Hydrogen bonding plays an important role in many of the molecules that make up living things.

Chemistry of Life Hydrogen Bonding Section 1

Chemistry of Life Unit 3 – Chemistry of Life SECTION 2 Section 1

Chemistry of Life Section 1 Properties of Water • Water has many unique properties that make it an important substance for life. • Most of the unique properties of water result because water molecules form hydrogen bonds with each other. • When water freezes, the crystal structure formed due to hydrogen bonding makes ice less dense than liquid water.

Chemistry of Life Section 1 Properties of Water, continued • Water can absorb a large amount of heat without changing temperature. This property can help organisms maintain a constant internal temperature. • The attraction of particles of the same substance, such as water, is called cohesion. Cohesion keep water from evaporating easily; thus, water is a liquid at ordinary temperatures. • Water molecules also stick to other polar molecules. This attraction between particles of different substances is called adhesion.

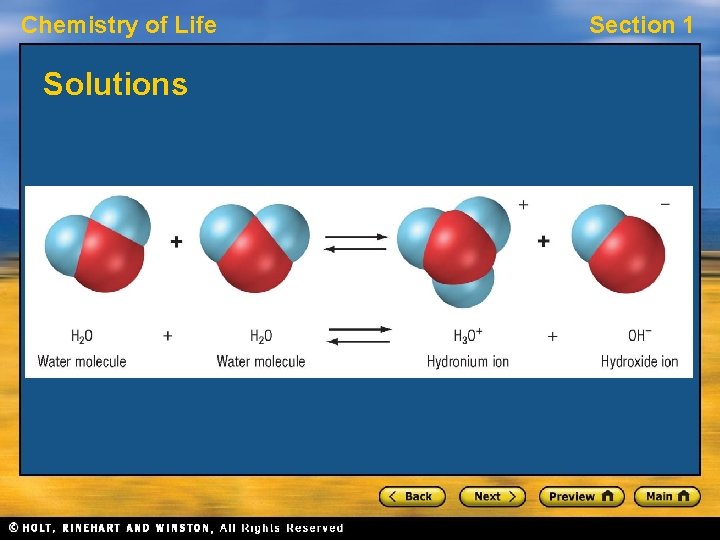
Chemistry of Life Section 1 Solutions • A solution is a mixture in which ions or molecules of one or more substances are evenly distributed in another substance. • Many substances are transported throughout living things as solutions of water. Dissolved substances can move more easily within and between cells. • Water dissolves many ionic and polar substances but does not dissolve nonpolar substances.

Chemistry of Life Section 1 Solutions, continued Acids and Bases • Some water molecules break apart to form hydronium and hydroxide ions. • In pure water, hydronium and hydroxide ions are present in equal numbers. • Acids and bases are compounds that change the balance of these ions.

Chemistry of Life Solutions Section 1

Chemistry of Life Section 1 Solutions, continued Acids and Bases • Acids are compounds that form extra hydronium ions when dissolved in water. • Bases are compounds that form extra hydroxide ions when dissolved in water. • When acids and bases are mixed, the extra hydronium and hydroxide ions react to form water.

Chemistry of Life Section 1 Solutions, continued p. H and Buffers • p. H is a measure of how acidic or basic a solution is. • Pure water has a p. H of 7. Acidic solutions have a p. H below 7, and basic solutions have a p. H above 7.

Chemistry of Life Section 1 Solutions, continued p. H and Buffers • The p. H of solutions in living things must be stable. • For a stable p. H to be maintained, the solutions in living things contain buffers. • A buffer is a substance that reacts to prevent p. H changes in a solution.

Chemistry of Life Unit 3 – Chemistry of Life SECTION 3 Section 1

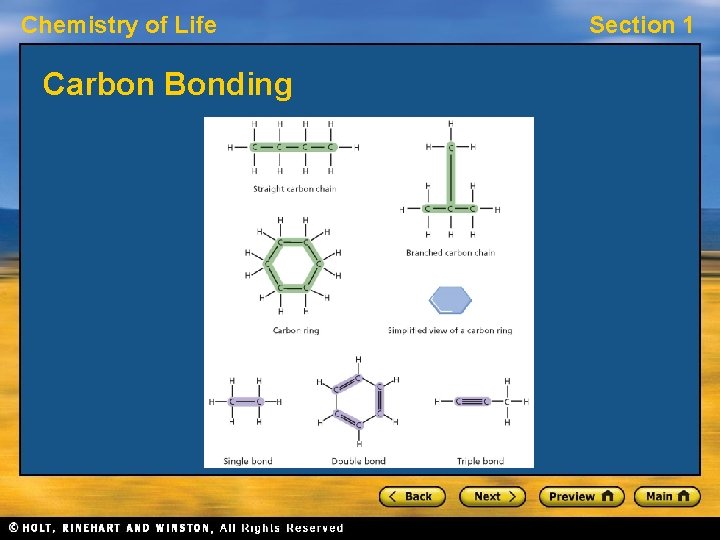
Chemistry of Life Section 1 Building Blocks of Cells • The parts of a cell are made up of large, complex molecules, often called biomolecules. • Large, complex biomolecules are built from a few smaller, simpler, repeating units arranged in an extremely precise way. • The basic unit of most biomolecules contain atoms of carbon. Carbon atoms can form covalent bonds with as many as four other atoms.

Chemistry of Life Carbon Bonding Section 1

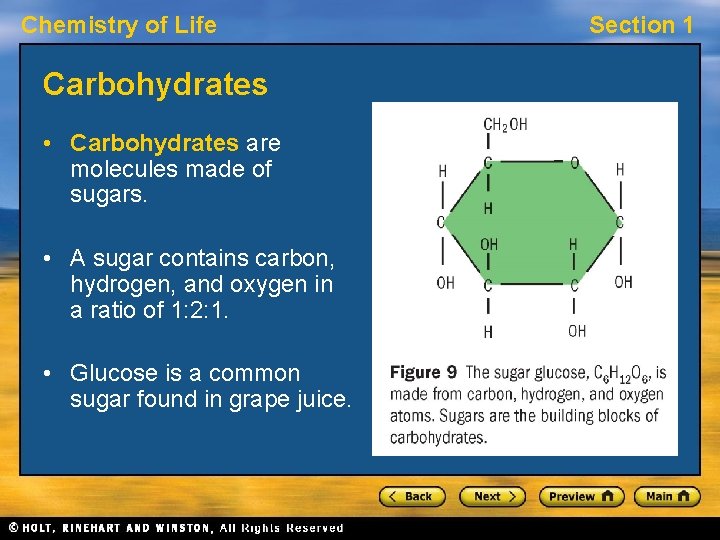
Chemistry of Life Carbohydrates • Carbohydrates are molecules made of sugars. • A sugar contains carbon, hydrogen, and oxygen in a ratio of 1: 2: 1. • Glucose is a common sugar found in grape juice. Section 1

Chemistry of Life Section 1 Carbohydrates, continued • Glucose is a monosaccharide, or “single sugar. ” • Two sugars can be linked to make a disaccharide. • Many sugars can be linked to make a polysaccharide. • Monosaccharides and disaccharides are considered simple carbohydrates. Polysaccharides are considered complex carbohydrates.

Chemistry of Life Section 1 Carbohydrates, continued • Cells use carbohydrates for sources of energy, structural materials, and cellular identification. • Carbohydrates are a major source of energy for many organisms, including humans.

Chemistry of Life Section 1 Lipids • Lipids are another class of biomolecules, which includes fats, phospholipids, steroids, and waxes. • Lipids consist of chains of carbon atoms bonded to each other and to hydrogen atoms. This structure makes lipids repel water. • The main functions of lipids include storing energy and controlling water molecules.

Chemistry of Life Lipids, continued • The main purpose of fats is to store energy. • Fats can store energy even more efficiently than carbohydrates. Section 1

Chemistry of Life Section 1 Proteins • Proteins are chains of amino acids that twist and fold into certain shapes that determine what the proteins do. • There are many types of proteins that perform many types of functions. • Proteins may be involved in structure, support, movement, communication, transportation, and carrying out chemical reactions.

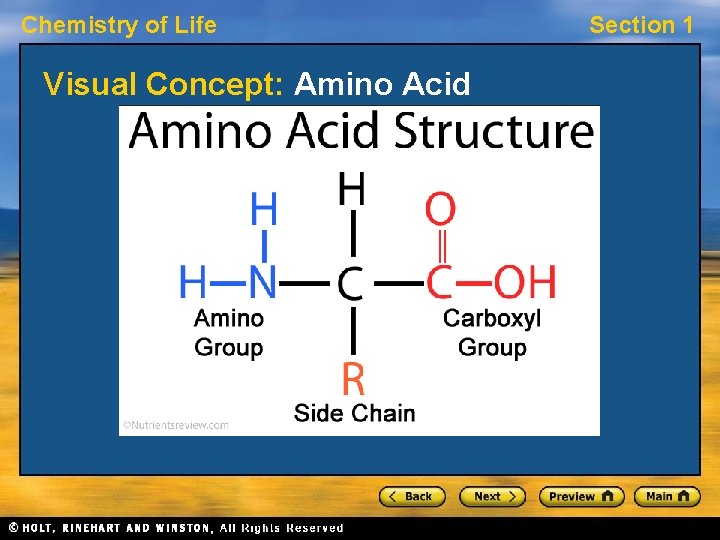
Chemistry of Life Section 1 Proteins, continued Amino Acids • A protein is a molecule made up of amino acids, building blocks that link to form proteins. • Every amino acid has an amino group and a carboxyl group. Units of amino acids can form links called peptide bonds. • The side group gives an amino acid its unique properties. Twenty different amino acids are found in proteins.

Chemistry of Life Visual Concept: Amino Acid Section 1

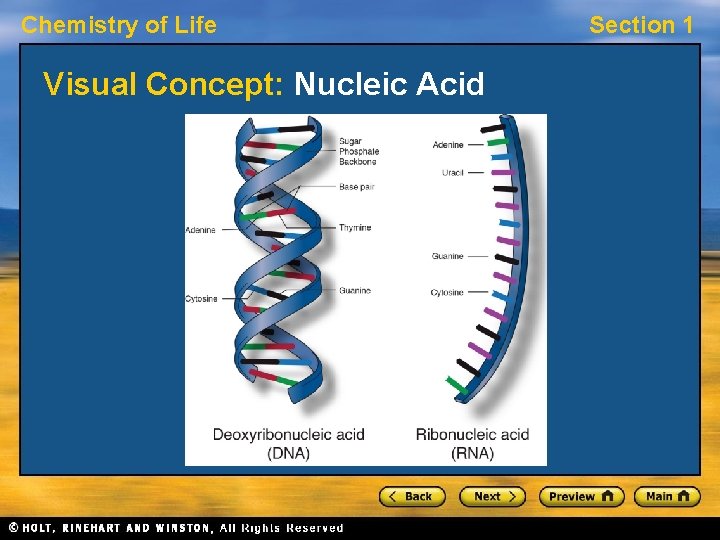
Chemistry of Life Section 1 Nucleic Acids • A nucleic acid is a long chain of nucleotide units. • A nucleotide is a molecule made up of three parts: a sugar, a base, and a phosphate group. • Nucleotides of deoxyribonucleic acid, or DNA, contain the sugar deoxyribose. • Nucleotides of ribonucleic acid, or RNA, contain the sugar ribose.

Chemistry of Life Visual Concept: Nucleic Acid Section 1

Chemistry of Life Section 1 Nucleic Acids, continued Hereditary Information • DNA molecules act as “instructions” for the processes of an organism’s life. • DNA consists of two strands of nucleotides that spiral around each other. • RNA also interacts with DNA to help decode the information. • Nucleic acids store and transmit hereditary information.

Chemistry of Life Section 1 Nucleic Acids, continued Energy Carriers • Some single nucleotides have other important roles. • Adenosine triphosphate, or ATP, is a nucleotide that has three phosphate groups and supplies energy to cells. • Energy is released in the reaction that breaks off the third phosphate group. • Other single nucleotides transfer electrons or hydrogen atoms for other life processes.

Chemistry of Life Section 1 Summary • All matter is made up of atoms. An atom has a positively charged nucleus surrounded by a negatively charged electron cloud. • Chemical bonds form between groups of atoms because most atoms became stable when they have eight electrons in the valence shell. • Polar attractions and hydrogen bonds are forces that play an important role in many of the molecules that make up living things.

Chemistry of Life Section 1 Summary • The hydrogen bonding between water molecules explains many of the unique properties that make water an important substance for life. • Acids and bases change the concentration of hydronium ions in aqueous solutions. The p. H of solutions in living things must be stable.

Chemistry of Life Section 1 Summary • Large, complex biomolecules are built from a few smaller, simpler, repeating units arranged in an extremely precise way. • Cells use carbohydrates for sources of energy, structural materials, and cellular identification. • The main functions of lipids include storing energy and controlling water movement

Chemistry of Life Section 1 Summary, continued • Proteins are chains of amino acids that twist and fold into shapes that determine what the protein does. • Nucleic acids store and transmit hereditary information.