Chemistry of Life Macromolecules Carbon Backbone of life
Chemistry of Life Macromolecules

Carbon Backbone of life • Organic Compounds – Contain carbon • Inorganic Compounds – Do not contain carbon

Making and Breaking Molecules • Breakdown of = Hydrolysis (just add water) • Formation of = Dehydration Synthesis (water is a byproduct)
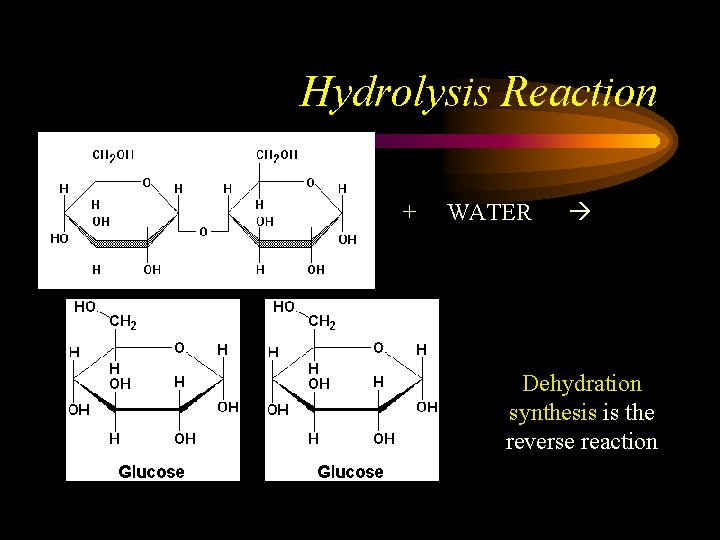
Hydrolysis Reaction + + WATER Dehydration synthesis is the reverse reaction

Carbohydrates • • Contain C, H, O Sugars & Starches Always found in rings Important source of energy 1. Monosaccharides – 1 ring sugars 2. Disaccharides – 2 sugar rings 3. Polysaccharides – many sugar rings
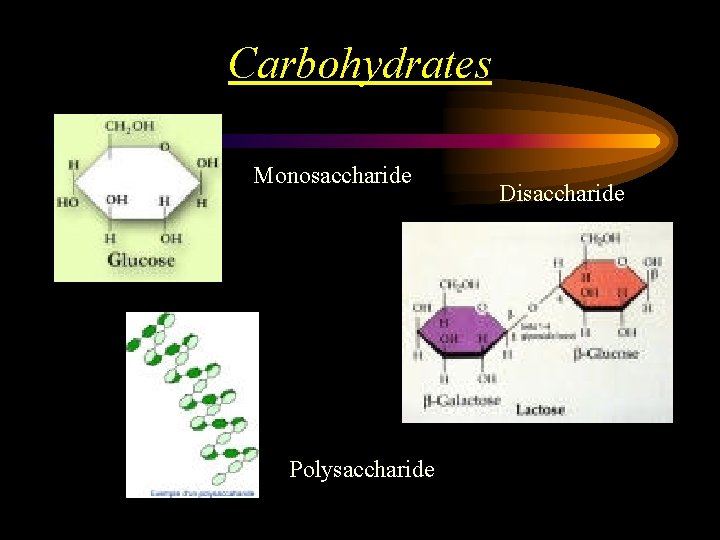
Carbohydrates Monosaccharide Polysaccharide Disaccharide
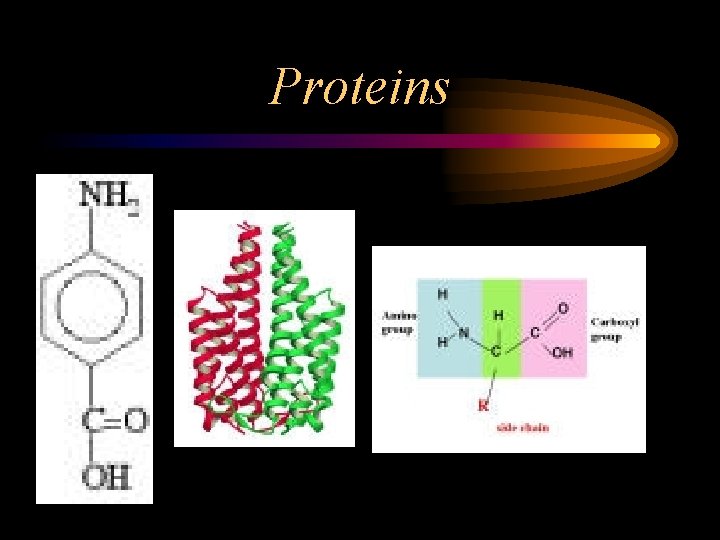
Proteins • Contain C, H, O, N • Made up of amino acids (a. a. ) – 20 – Amine Group (NH 2) – Carboxyl Group (COOH) – An R Group (side chain) • Major structural & functional component of living organisms
Proteins
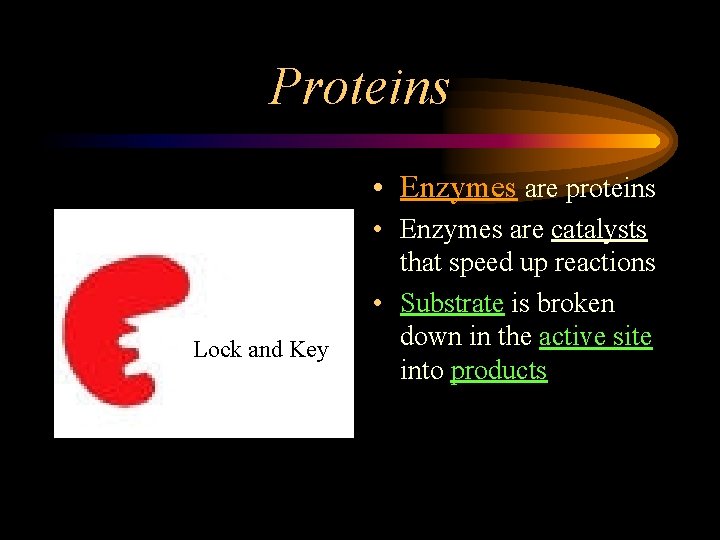
Proteins • Enzymes are proteins Lock and Key • Enzymes are catalysts that speed up reactions • Substrate is broken down in the active site into products
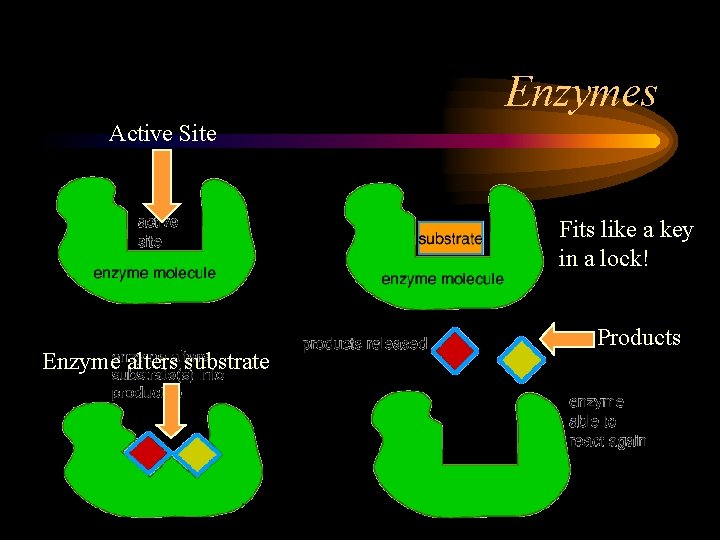
Enzymes Active Site Fits like a key in a lock! Enzyme alters substrate Products
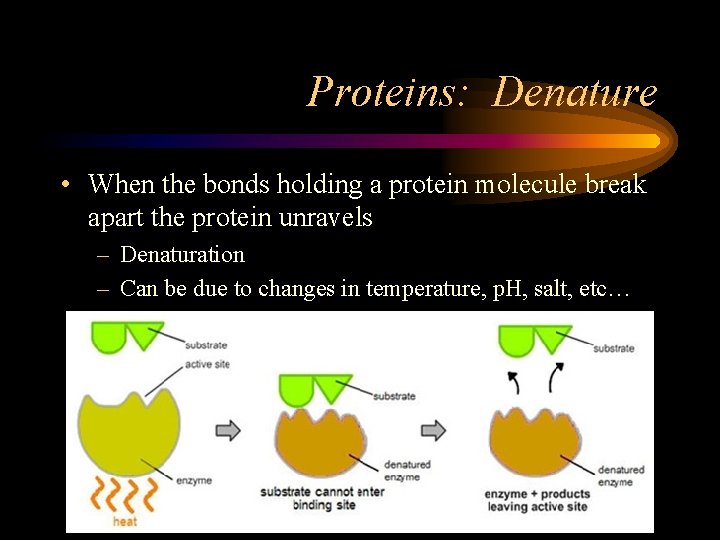
Proteins: Denature • When the bonds holding a protein molecule break apart the protein unravels – Denaturation – Can be due to changes in temperature, p. H, salt, etc…

Lipids • Consist of C, H, O • Fats, oils, steroids, phospholipids • Structural component cell membranes, insulation, energy storage • Triglyceride – glycerol + 3 fatty acid chains
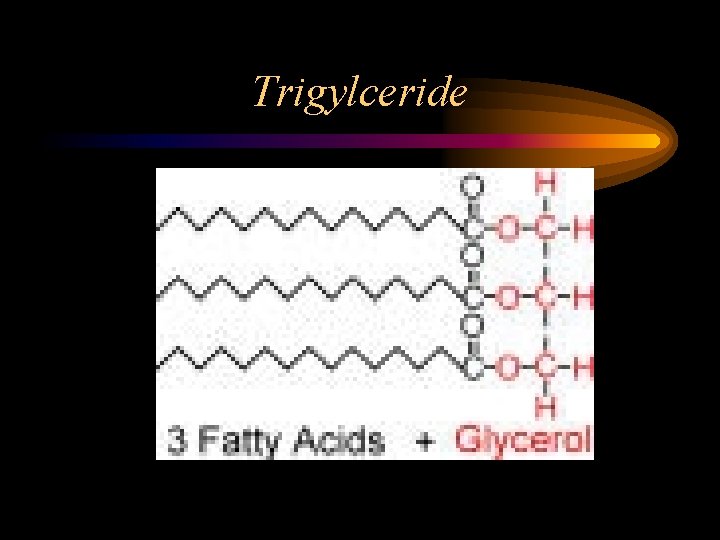
Trigylceride
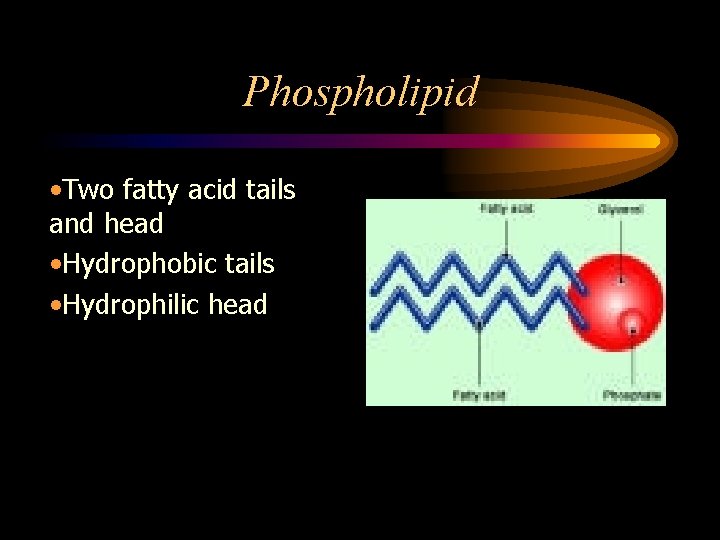
Phospholipid • Two fatty acid tails and head • Hydrophobic tails • Hydrophilic head

Nucleic Acid • Consist of C, H, O, N, P • Made up of nucleotides – 5 Carbon Sugar – Nitrogen Base – Phosphate Group • DNA (double stranded) & RNA (single stranded)
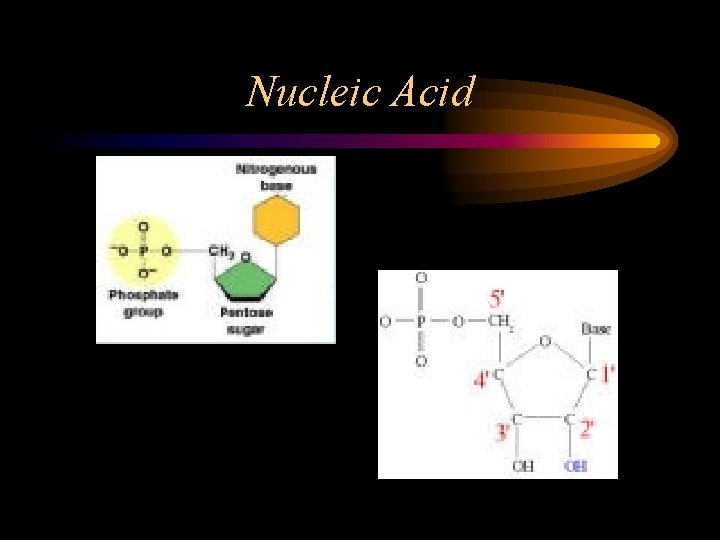
Nucleic Acid
- Slides: 16