Chemistry of Life BIOCHEMISTRY SECTION 1 21 8
Chemistry of Life BIOCHEMISTRY: SECTION 1. 21. 8 MAAP WORKBOOK
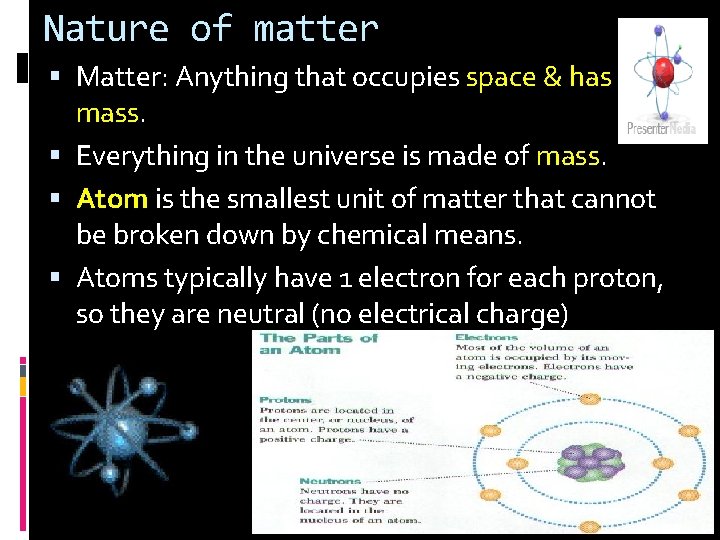
Nature of matter Matter: Anything that occupies space & has mass. Everything in the universe is made of mass. Atom is the smallest unit of matter that cannot be broken down by chemical means. Atoms typically have 1 electron for each proton, so they are neutral (no electrical charge)
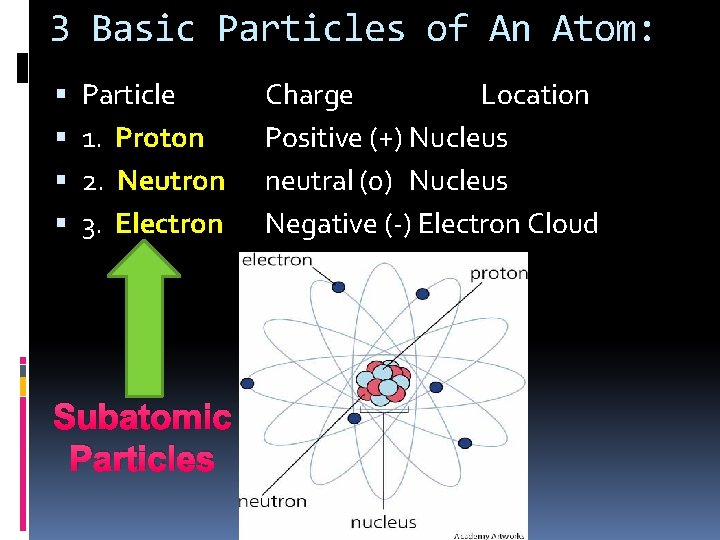
3 Basic Particles of An Atom: Particle 1. Proton 2. Neutron 3. Electron Subatomic Particles Charge Location Positive (+) Nucleus neutral (0) Nucleus Negative (-) Electron Cloud
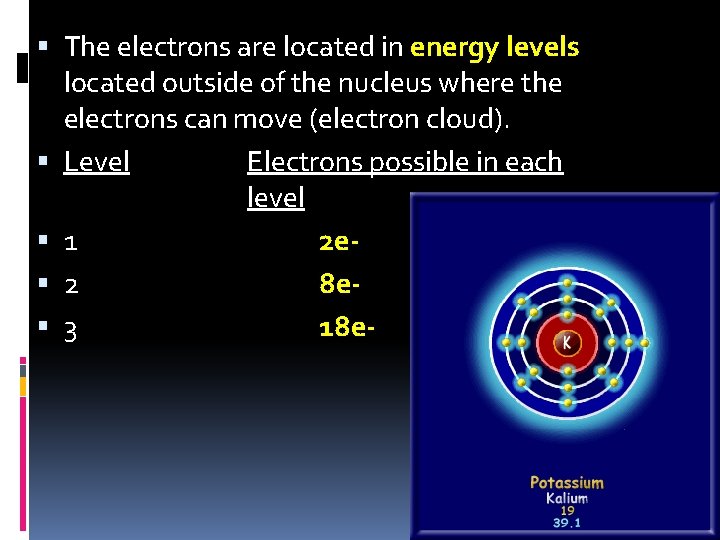
The electrons are located in energy levels located outside of the nucleus where the electrons can move (electron cloud). Level Electrons possible in each level 1 2 e 2 8 e 3 18 e-
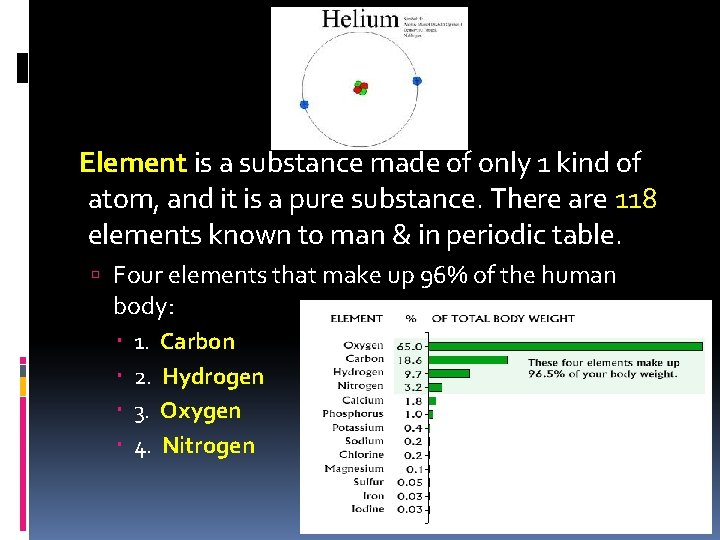
Element is a substance made of only 1 kind of atom, and it is a pure substance. There are 118 elements known to man & in periodic table. Four elements that make up 96% of the human body: 1. 2. 3. 4. Carbon Hydrogen Oxygen Nitrogen

Chemical formulas When elements react to form molecules, the molecule is abbreviated by using the chemical symbol of the element The subscript indicates how many atoms of each element is in one molecule of the compound Chemical formulas are the combination of chemical symbols and subscripts when elements react to form molecules or compounds. Ex: 6 CO 2 + 6 H 2 O+ sunlight C 6 H 12 O 6 + 6 O 2
A force that joins atoms is called a bond. There are 2 main types of bonds: 1. Covalent bonds 2. Ionic bonds Ionic and covalent compounds are alike in that they both fill outer electron levels.
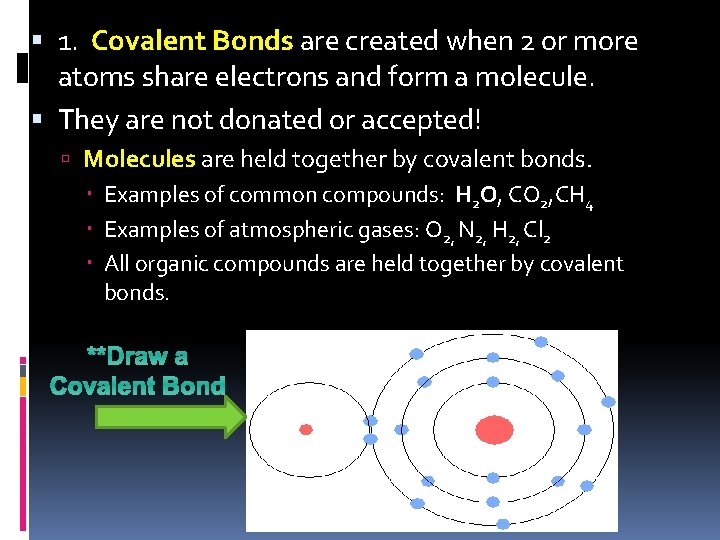
1. Covalent Bonds are created when 2 or more atoms share electrons and form a molecule. They are not donated or accepted! Molecules are held together by covalent bonds. Examples of common compounds: H 2 O, CO 2, CH 4 Examples of atmospheric gases: O 2, N 2, H 2, Cl 2 All organic compounds are held together by covalent bonds. **Draw a Covalent Bond

Draw a covalent bond
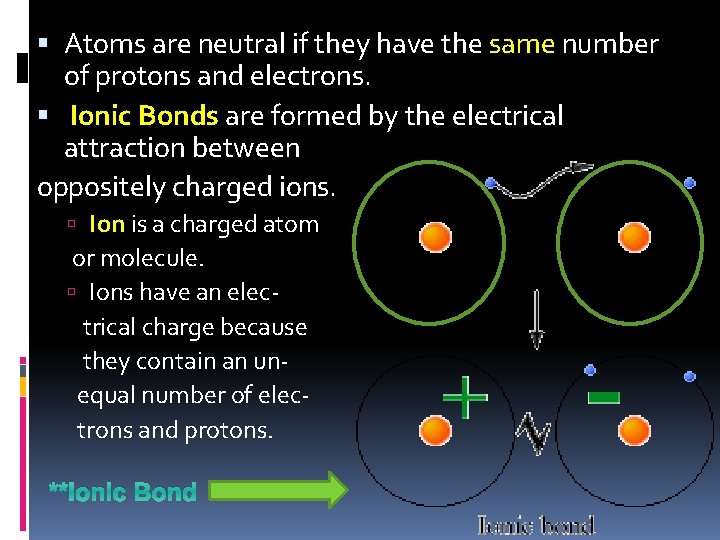
Atoms are neutral if they have the same number of protons and electrons. Ionic Bonds are formed by the electrical attraction between oppositely charged ions. Ion is a charged atom or molecule. Ions have an electrical charge because they contain an unequal number of electrons and protons. **Ionic Bond

Draw an ionic bond
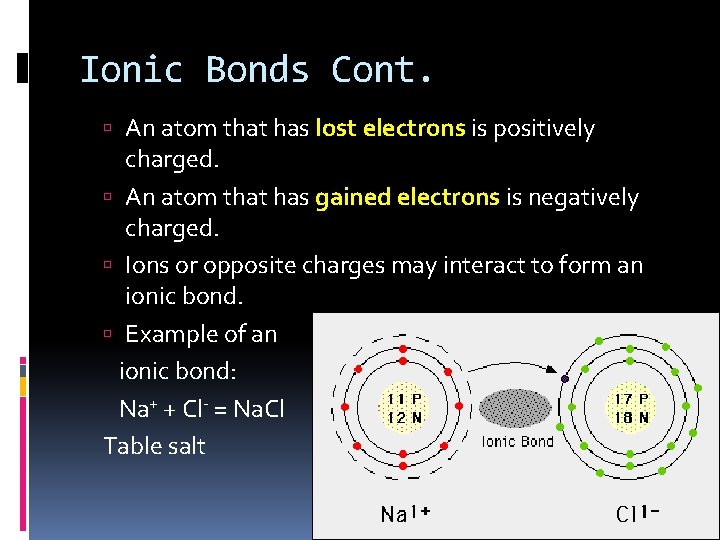
Ionic Bonds Cont. An atom that has lost electrons is positively charged. An atom that has gained electrons is negatively charged. Ions or opposite charges may interact to form an ionic bond. Example of an ionic bond: Na+ + Cl- = Na. Cl Table salt
Inorganic vs organic molecules Inorganic compounds do NOT contain carbon are not made by living organisms. Examples: salts, metals, atmospheric gases
Chemistry of cells Organic compounds contain carbon atoms that are covalently bonded to other elements, typically hydrogen, oxygen and other carbon atoms. All organic compounds contain the element carbon. Carbon is the essential element that all life depends on. Carbon can form up to 4 covalent bonds with other molecules. Carbon’s ability to form covalent bonds is important in allowing for a wider variety of organic molecules. Living things require such a variety to carry out life processes.
Biomolecules are organic molecules produced by living organisms. Ex: vitamins, hormones, ATP Macromolecules are special biomolecules that are long and complex. Macromolecules can be called macronutrients, organic compounds, or organic macromolecules These organic macromolecules are made of different combinations of the following elements: Carbon (C), Hydrogen (H), Oxygen (O), Nitrogen (N), Phosphorus (P), & Sulfur (S). CHONPS

Four Classes of Organic Macromolecules Found in Living Things: 1. 2. 3. 4. Carbohydrates Lipids nucleic acids proteins Without these compounds cells could not function.
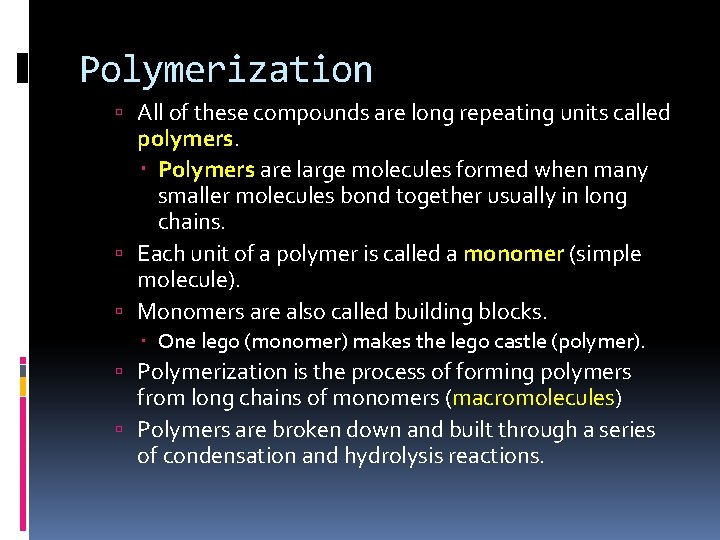
Polymerization All of these compounds are long repeating units called polymers. Polymers are large molecules formed when many smaller molecules bond together usually in long chains. Each unit of a polymer is called a monomer (simple molecule). Monomers are also called building blocks. One lego (monomer) makes the lego castle (polymer). Polymerization is the process of forming polymers from long chains of monomers (macromolecules) Polymers are broken down and built through a series of condensation and hydrolysis reactions.
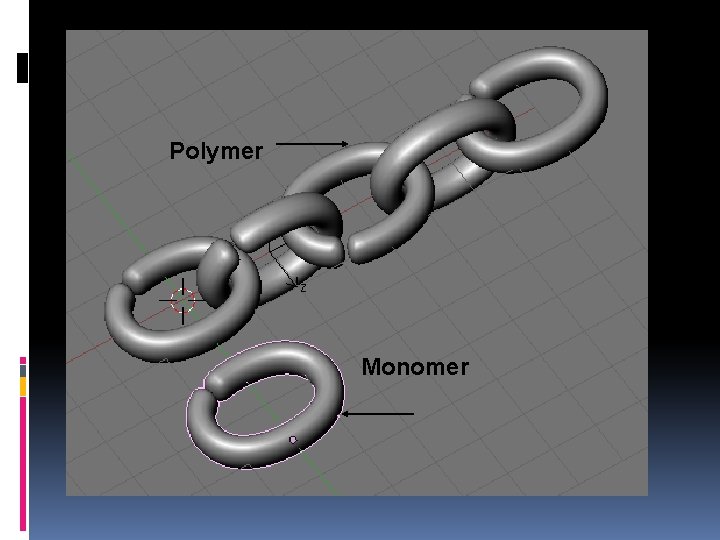
Polymer Monomer

Condensation reactions also known as dehydration synthesis reactions Form one water molecule each time you add a monomer Builds polymers from monomers using water

Hydrolysis reactions Uses water to break apart polymers Splits water into hydrogen (H+) and hydroxide (OH-). Used to break a bond between monomers. Breaks down polymers to form monomers Breaks down macromolecules into smaller molecules Sometimes used to obtain energy

Four Organic macromolecules: 1. Carbohydrates are organic compounds made of carbon, hydrogen, and oxygen atoms in 1: 2: 1 ratio. Example: C 6 H 12 O 6 All carbohydrates are made of carbon, hydrogen, and oxygen. (CHO) They are the main source of energy for living things, and they are found in most foods—like fruits, vegetables, and grains. Most energy that is used in the human body is stored as carbohydrates. They are the most abundant biomolecule on Earth.

Functions of carbohydrates: Primary source of energy for living things (sugars) Found in cell structures (cell membrane & cytoplasm), serve structural purposes in plants (cell walls) and some animals Form parts of other macromolecules (nucleic acids) Broken down in cells to provide Carbon to make other macromolecules Provide fiber to aid in digestion in animal diets

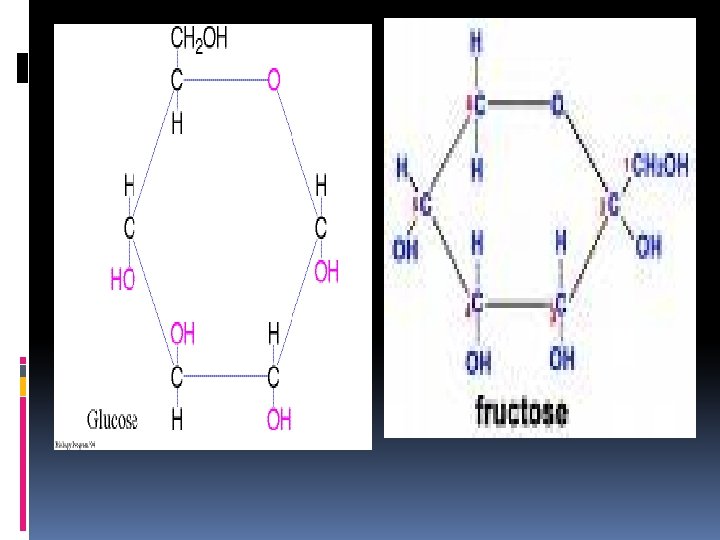
There are 3 types of carbohydrates: 1. Monosaccharides or simple sugars. They are the building blocks of carbohydrates and provide immediate energy or can be converted to polysaccharides for later use. (CH 2 O) Examples of monosaccharides: 1. Glucose is manufactured by plants during photosynthesis. It is the main source of energy for plants and animals, so it’s the most important. 2. Fructose is found in fruits and is sweet. These have the same molecular formulas, C 6 H 12 O 6, but different structural formulas, which make them isomers. Galactose-found in milk
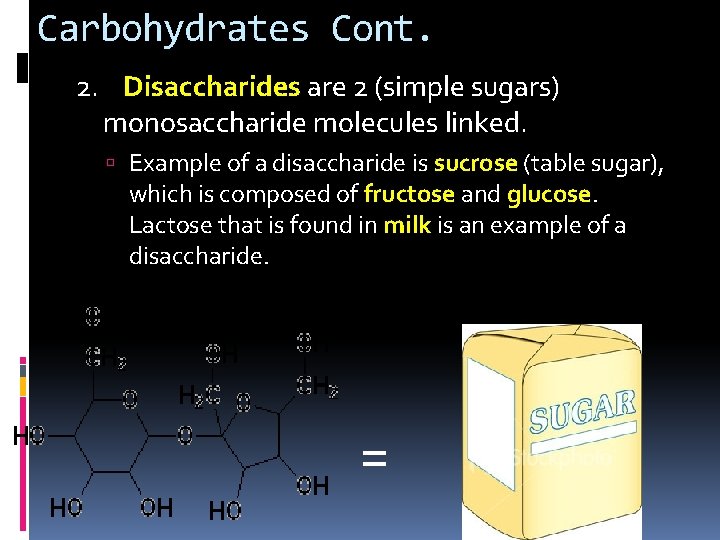
Carbohydrates Cont. 2. Disaccharides are 2 (simple sugars) monosaccharide molecules linked. Example of a disaccharide is sucrose (table sugar), which is composed of fructose and glucose. Lactose that is found in milk is an example of a disaccharide. =

3. Polysaccharides are composed of many monosaccharide subunits. Poly= many; saccharide= sugar Polysaccharides function as storehouses of the energy contained sugars. When plants and animals need energy they break down polysaccharides into monosaccharides (like glucose) for energy.

Examples of polysaccharides that store energy: 1. Starch which is made by plants (like potatoes). When the plant makes glucose during photosynthesis, excess glucose can be stored as starch. Starch is one of the most important polysaccharides. Energy is passed from potato to the person eating it primarily by the energy stored in starch molecules. 2. Glycogen which is made by animals when they eat more sugar than they immediately need. Both starch and glycogen are made of hundreds of linked glucose molecules to be stored for later use. Glycogen is mainly stored in the liver, but some can be stored in muscle cells. It is broken down when energy is needed. Starch Glycogen in Liver Cells
3. Cellulose is a polysaccharide that provides structural support for plants. It gives plant cell walls strength instead of providing energy. It is a major component of wood and cotton; can be found in fruits and vegetables. Humans cannot digest cellulose but it aids in digestion by slowing the absorption of nutrients. It also aids elimination by absorbing water and speeding the passage of unused food through the large intestine. 4. Chitin is a polysaccharide used for structural support in fungi cell walls and in exoskeletons of certain animals (crabs, insects) that causes them to “crunch”. Cellulose
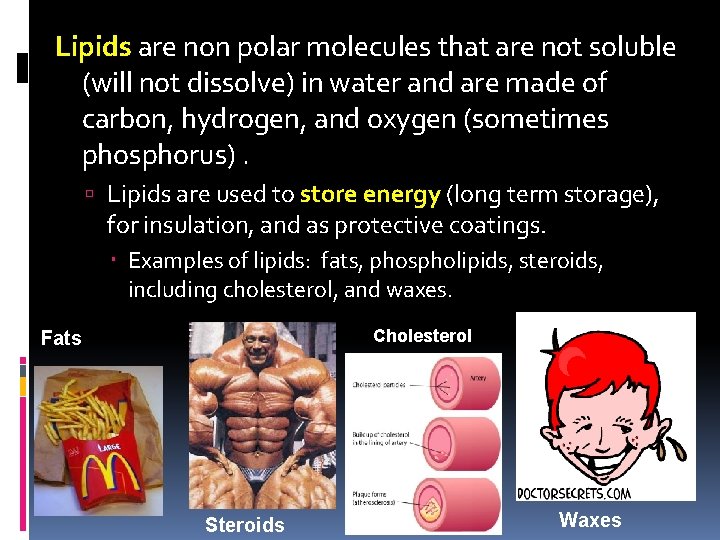
Lipids are non polar molecules that are not soluble (will not dissolve) in water and are made of carbon, hydrogen, and oxygen (sometimes phosphorus). Lipids are used to store energy (long term storage), for insulation, and as protective coatings. Examples of lipids: fats, phospholipids, steroids, including cholesterol, and waxes. Cholesterol Fats Steroids Waxes
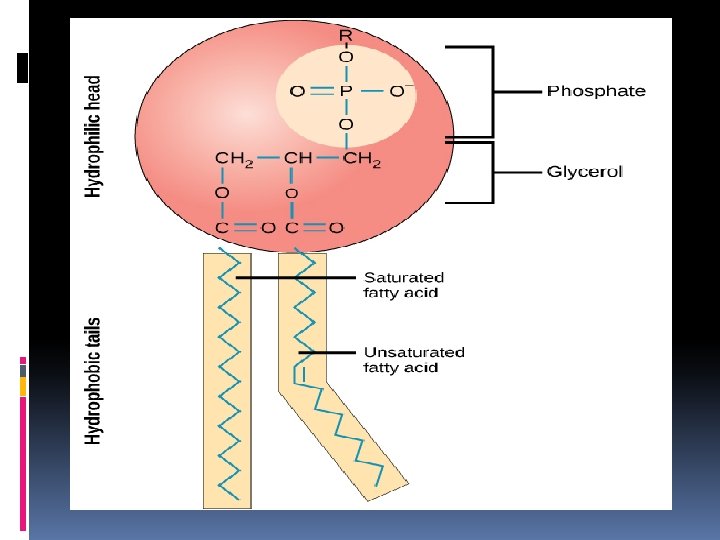
Structure of Lipids The monomers of lipids are fatty acids Fatty acids are organic acids made of long chains of hydrocarbons containing an even number of carbon atoms with a carboxyl group (-COOH) at the end of the chain. The carboxyl group makes them acidic. Fats are also called triglycerides and are made of a glycerol molecule bonded to 3 fatty acids. Fatty acids may be saturated or unsaturated. Fats are lipids that store energy.
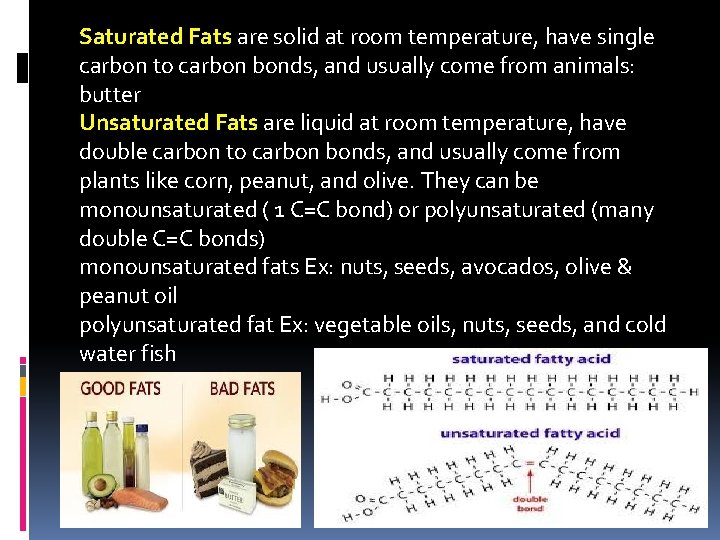
Saturated Fats are solid at room temperature, have single carbon to carbon bonds, and usually come from animals: butter Unsaturated Fats are liquid at room temperature, have double carbon to carbon bonds, and usually come from plants like corn, peanut, and olive. They can be monounsaturated ( 1 C=C bond) or polyunsaturated (many double C=C bonds) monounsaturated fats Ex: nuts, seeds, avocados, olive & peanut oil polyunsaturated fat Ex: vegetable oils, nuts, seeds, and cold water fish
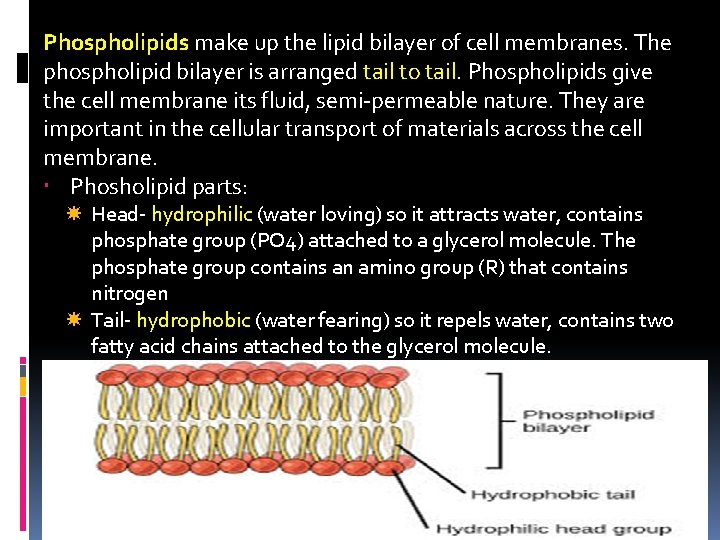
Phospholipids make up the lipid bilayer of cell membranes. The phospholipid bilayer is arranged tail to tail. Phospholipids give the cell membrane its fluid, semi-permeable nature. They are important in the cellular transport of materials across the cell membrane. Phosholipid parts: Head- hydrophilic (water loving) so it attracts water, contains phosphate group (PO 4) attached to a glycerol molecule. The phosphate group contains an amino group (R) that contains nitrogen Tail- hydrophobic (water fearing) so it repels water, contains two fatty acid chains attached to the glycerol molecule.

Steroids also make up a structural component of cell membranes and are used to make hormones. Steroids are also used to regulate several essential biological processes, like metabolism, immunity, and reproduction. Steroids contain 4 carbon rings. Two types of steroids: Cholesterol: used to make steroid hormones, major component of cell membranes, and found in other structures in cells. Hormones: chemical messengers. Ex: estrogen, testosterone, progesterone (sex hormones).

Waxes are a type of highly waterproof lipid. They are made of fatty acids and other organic compounds. In plants, wax forms a protective coating on the outer surfaces, for example on the leaves (shiny). Also, certain palm trees make carauba wax to wax cars. In animals, wax forms protective layers, for example ear wax in animals and bees make beeswax.

Functions of lipids: Fats: long term energy storage, source of fatty acids to make other lipids, insulation against cold, protection around organs Phospholipids: major component of cell membranes Steroids: regulates several types of essential biological processes and forms certain hormones Wax: provides a waterproof covering

3. Nucleic Acids are in all of your cells. Nucleic acids are made of carbon, hydrogen, oxygen, nitrogen, and phosphorous (CHONP). The building blocks (monomers) of nucleic acids are nucleotides. A nucleic acid is a long chain of smaller molecules called nucleotides. A nucleotide has three parts; 1. a sugar 2. a base 3. a phosphate group (PO 4)
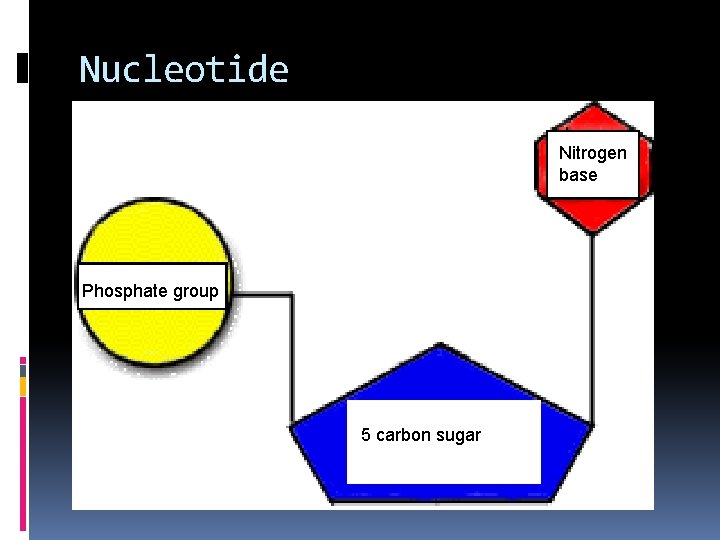
Nucleotide Nitrogen base Phosphate group 5 carbon sugar

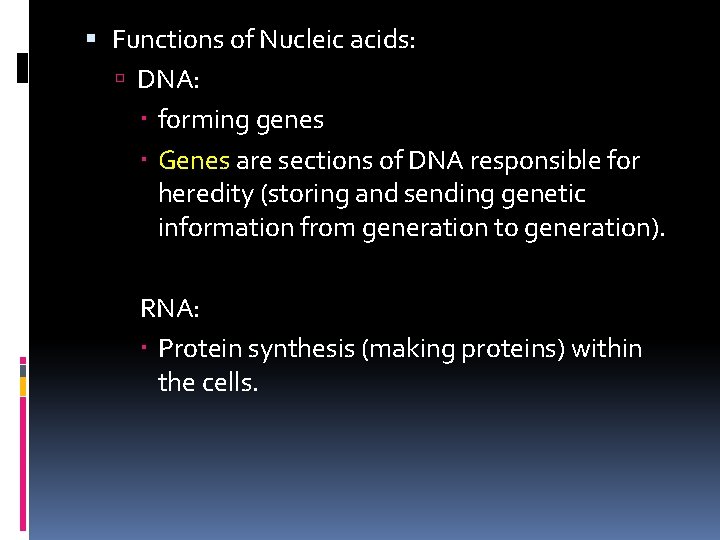
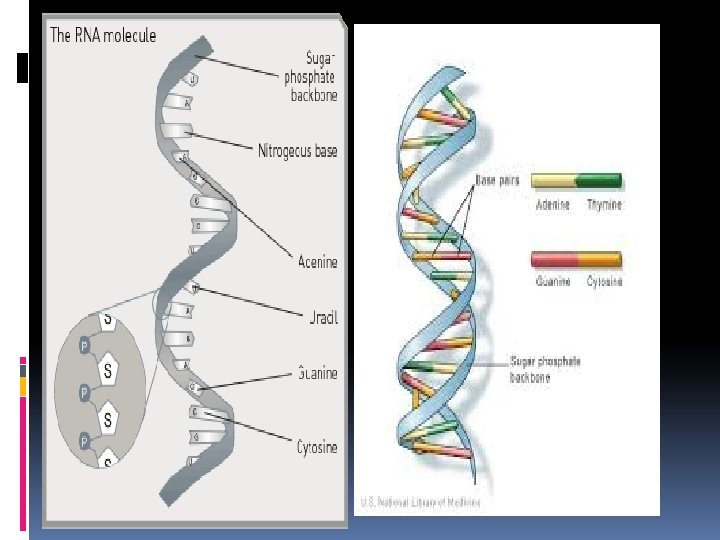
There are two types of nucleic acids: 1. DNA Deoxyribonucleic Acid Contains the sugar deoxyribose and nitrogenous bases: Adenine, Thymine, Guanine, and Cytosine. Erwin Chargaff, biochemist, found that there were equal amount of Adenine and Thymine and equal amounts of Cytosine and Guanine in DNA. Thus, indicating A pairs with T, and C pairs with G. DNA consists of 2 strands of nucleotides that spiral around each other. DNA is a molecule shaped like a spiral staircase (ladder) also known as a double helix. The 2 strands of a DNA molecule are held together by hydrogen bonds between two bases across from one another. The rungs (horizontal) of the ladder are formed by nitrogenous bases. The rails (vertical) of the ladder are formed by the sugars and phosphate groups.
2. RNA Ribonucleic Acid RNA consists or a single strand of nucleotides Contain ribose sugar and the nitrogen bases Adenine, Uracil, Cytosine, and Guanine.
Functions of Nucleic acids: DNA: forming genes Genes are sections of DNA responsible for heredity (storing and sending genetic information from generation to generation). RNA: Protein synthesis (making proteins) within the cells.
DNA

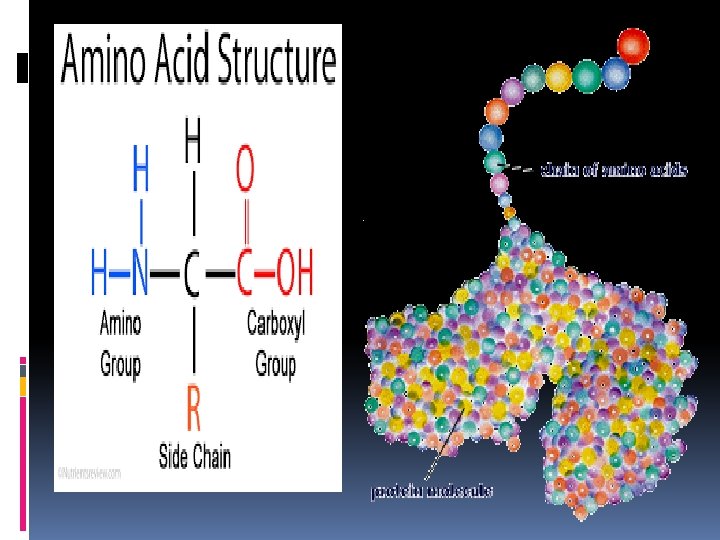
4. Proteins are a chain of molecules called amino acids linked together like parts on a necklace and are the most diverse group of macromolecules. The elements that make up proteins are carbon, hydrogen, oxygen, and nitrogen (CHON) Amino acids are the building blocks (monomers )of proteins. There are 20 different amino acids, which bond to each other by peptide bonds (covalent bonds formed between amino acids). Proteins are made of long chains of amino acids called polypeptides. Each amino acid has a central carbon atom with an attached hydrogen atom, an amino group, a carboxyl (COOH)group, and an R group (variable group). The R group can be any of 20 different atoms or groups of atoms, and varies with each amino acid. The amino group is 1 nitrogen and 2 hydrogen atoms on one end of the molecule.

How amino acids are arranged make different proteins. The arrangement, number, and type of amino acids are important because any change can change the protein’s shape and function. Examples of proteins include: some hormones, ATP, and enzymes.
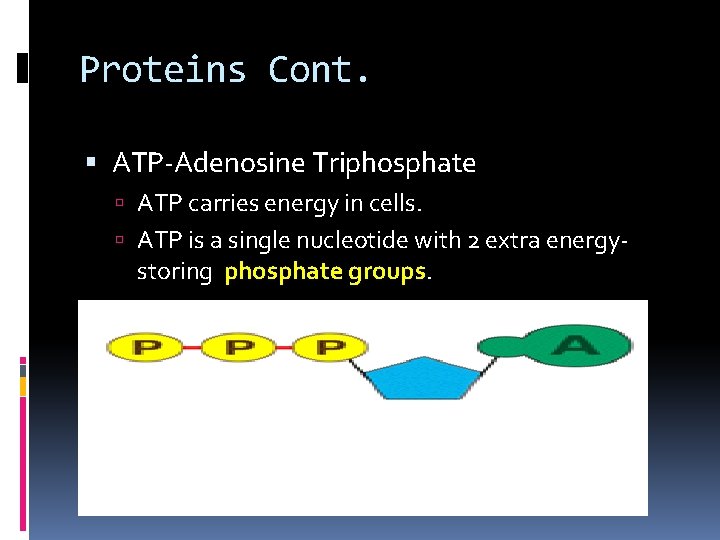
Proteins Cont. ATP-Adenosine Triphosphate ATP carries energy in cells. ATP is a single nucleotide with 2 extra energy- storing phosphate groups.

Hormones (chemical messengers) can be made of amino acids making them proteins. Remember: sex hormones are lipids (steroid hormones). Examples of hormones that are proteins: thyroid stimulating hormone and growth hormone. Some proteins called enzymes regulate chemical reactions in the body but remain unchanged by the reaction. Organisms rely on chemical reactions to survive. The complete hydrolysis of a protein would result in the formation of amino acids. Hydrolysis is a chemical reaction in which water is used to break down a compound.
Functions of proteins: Form main structural component of skeletal muscle, skin, cartilage, tendons, ligaments, horns, bones, hair, and feathers. Receptors that detect chemical signals so cells can respond to stimuli. Hormones acting as chemical messengers by sending signals for changes in cell activities. Important for the movement of muscles and cells. Antibodies to protect against diseases. Highly specialized as enzymes Help transport substances throughout the body Provide storage for elements like iron
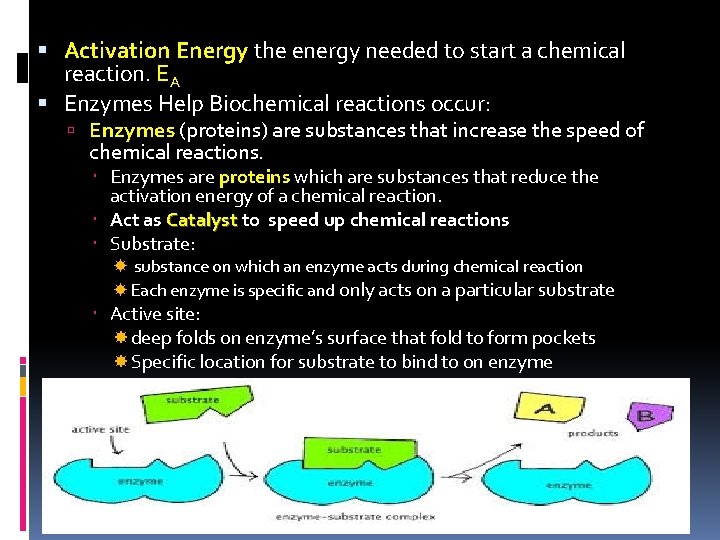
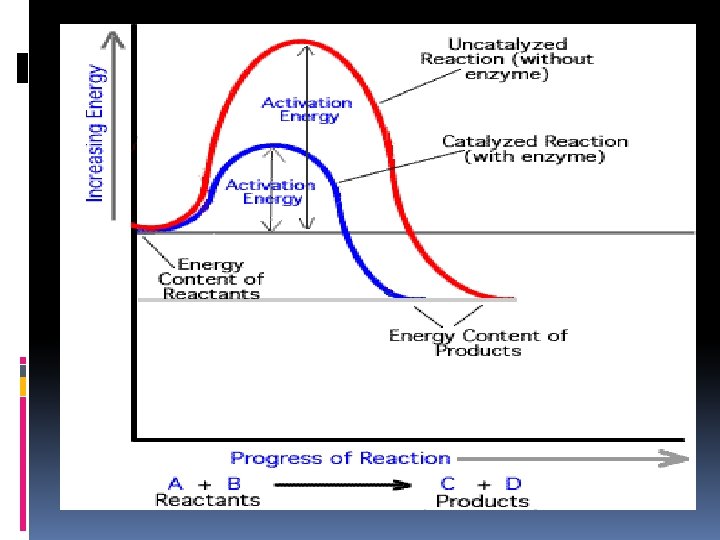
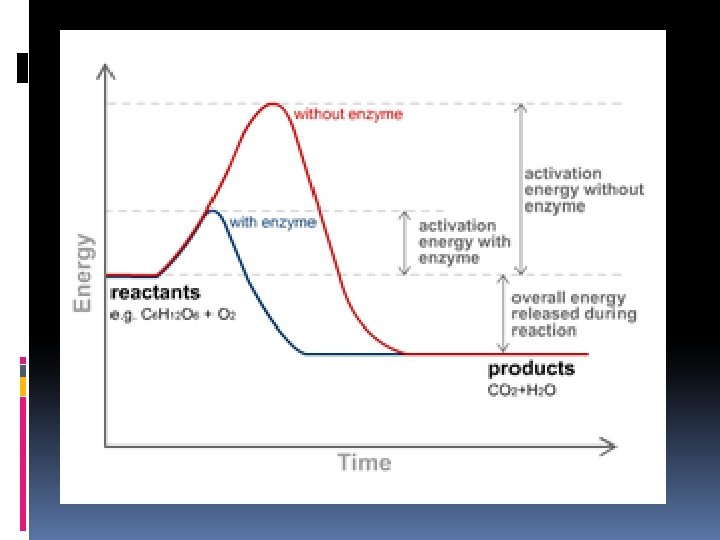
Activation Energy the energy needed to start a chemical reaction. EA Enzymes Help Biochemical reactions occur: Enzymes (proteins) are substances that increase the speed of chemical reactions. Enzymes are proteins which are substances that reduce the activation energy of a chemical reaction. Act as Catalyst to speed up chemical reactions Substrate: substance on which an enzyme acts during chemical reaction Each enzyme is specific and only acts on a particular substrate Active site: deep folds on enzyme’s surface that fold to form pockets Specific location for substrate to bind to on enzyme
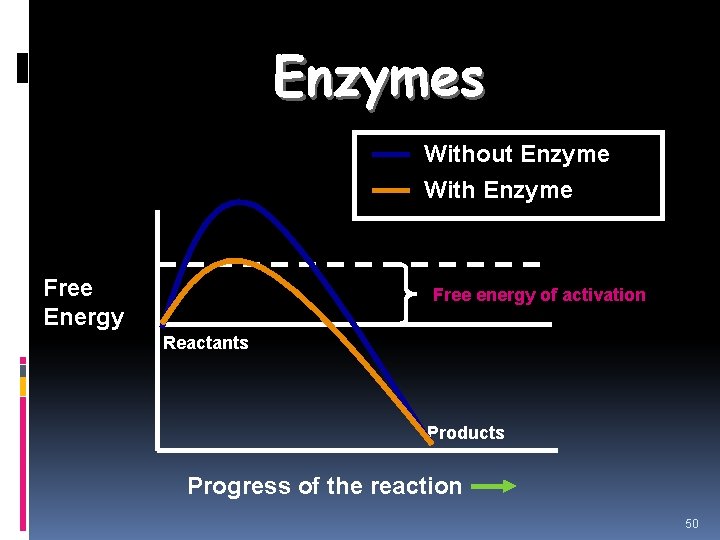
Enzymes Without Enzyme With Enzyme Free Energy Free energy of activation Reactants Products Progress of the reaction 50
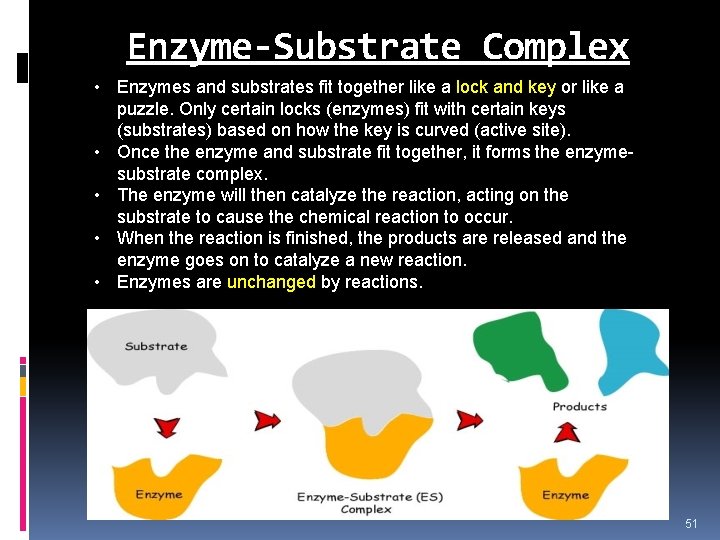
Enzyme-Substrate Complex • Enzymes and substrates fit together like a lock and key or like a puzzle. Only certain locks (enzymes) fit with certain keys (substrates) based on how the key is curved (active site). • Once the enzyme and substrate fit together, it forms the enzymesubstrate complex. • The enzyme will then catalyze the reaction, acting on the substrate to cause the chemical reaction to occur. • When the reaction is finished, the products are released and the enzyme goes on to catalyze a new reaction. • Enzymes are unchanged by reactions. 51
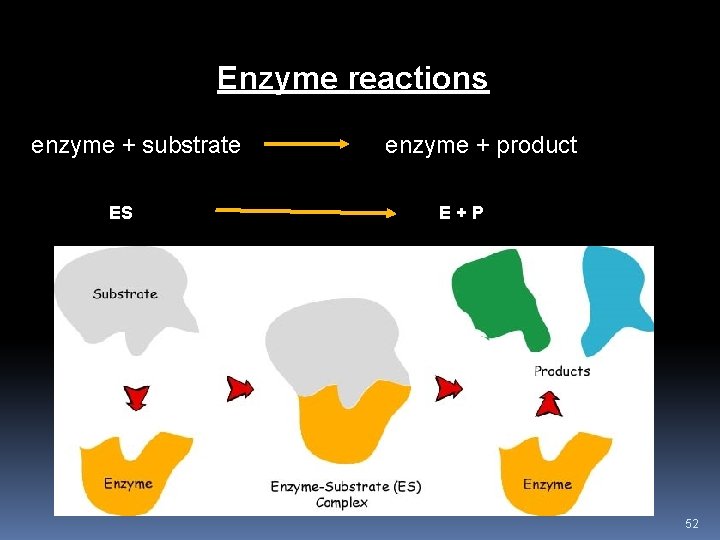
Enzyme reactions enzyme + substrate ES enzyme + product E+P 52
Types of enzymes: Humans produce metabolic and digestive enzymes. Humans obtain food enzymes from eating raw foods. 1. Metabolic enzymes: Enable cells to perform cellular reactions, which allow cells to make energy, repair tissues, and eliminate/neutralize wastes and toxic substances. 2. Digestive enzymes: Name of digestive enzyme indicates what it helps to digest. Help digest different macronutrients Allow the body to break down food in hours instead of days.
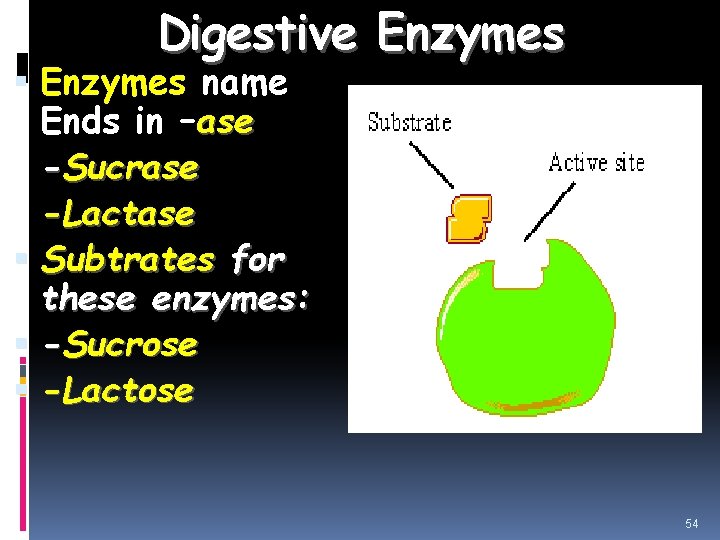
Digestive Enzymes name Ends in –ase -Sucrase -Lactase Subtrates for these enzymes: -Sucrose -Lactose 54

Ex: Lactase breaks the disaccharide lactose (milk sugar) into glucose and galactose. Ex: Sucrase breaks the disaccharide sucrose into glucose and fructose. 3. Food enzymes: Help break down foods we eat Destroyed at high temperatures, so only found in uncooked foods or supplements. Ex: lipase breaks down fat in avocado, protease helps digest protein in pineapple and papaya
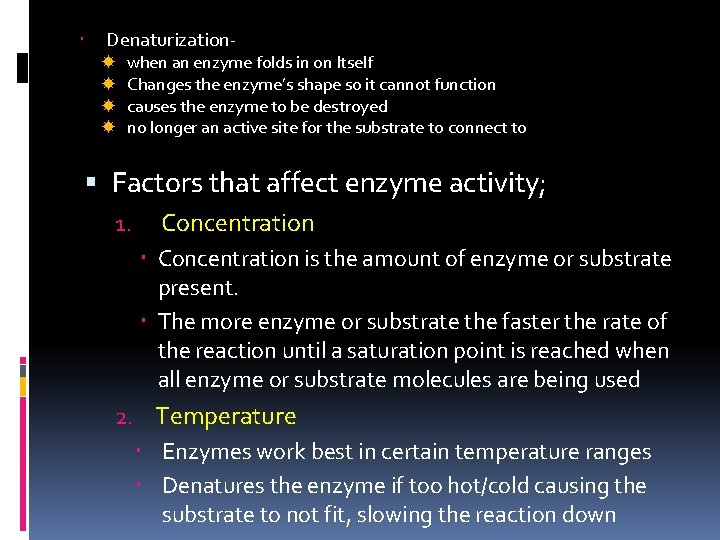
Denaturization when an enzyme folds in on Itself Changes the enzyme’s shape so it cannot function causes the enzyme to be destroyed no longer an active site for the substrate to connect to Factors that affect enzyme activity; 1. Concentration is the amount of enzyme or substrate present. The more enzyme or substrate the faster the rate of the reaction until a saturation point is reached when all enzyme or substrate molecules are being used 2. Temperature Enzymes work best in certain temperature ranges Denatures the enzyme if too hot/cold causing the substrate to not fit, slowing the reaction down
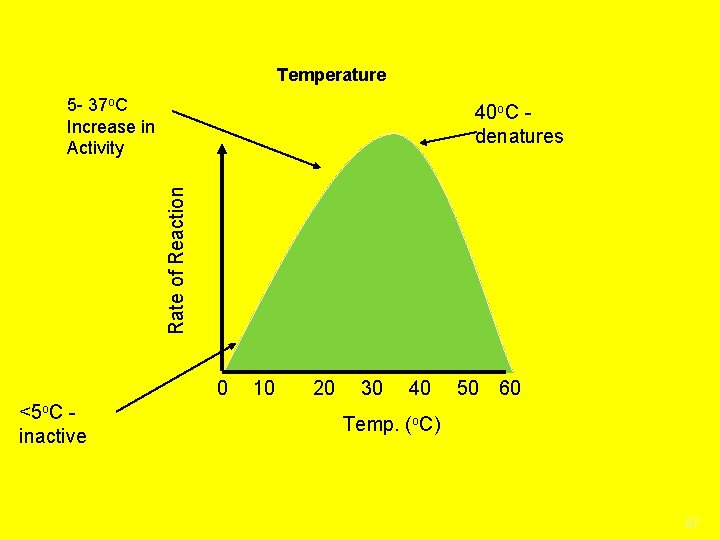
Temperature 5 - 37 o. C Increase in Activity Rate of Reaction 40 o. C denatures 0 <5 o. C inactive 10 20 30 40 50 60 Temp. (o. C) 57

3. Salinity: Salinity is the salt concentration of a solution Salts form ions when dissolved in water, so salinity is the number of ions in the water Some ions help enzyme activity and some can denature enzymes Most enzymatic reactions occur fastest with not too much or too little salt concentrations

4. p. H Enzymes work best if the p. H is not too high or low or they will denature p. H indicates acidity or alkalinity of a solution p. H is percent H+ ions in a solution Water separates into H+ and OH- ions 0 -14 Pure water is 7, neutral Acids 0 -6. 9 produce more H+ ions Bases/Alkaline 7. 1 -14 Produces more OH- ions
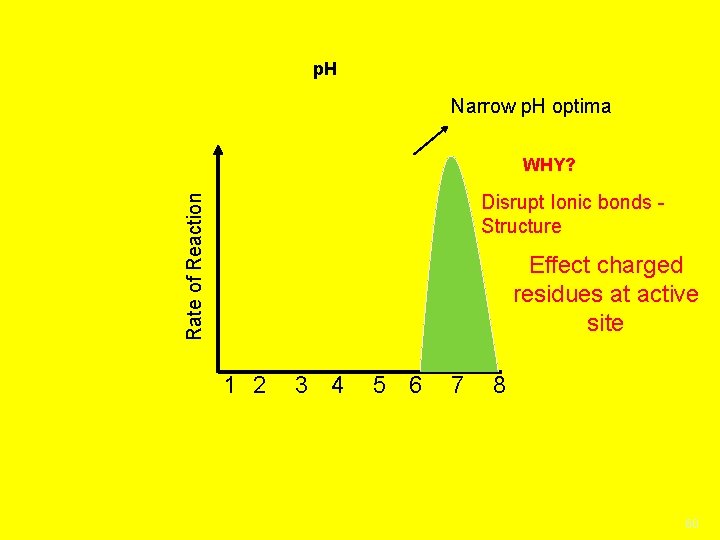
p. H Narrow p. H optima WHY? Rate of Reaction Disrupt Ionic bonds Structure Effect charged residues at active site 1 2 3 4 5 6 7 8 60

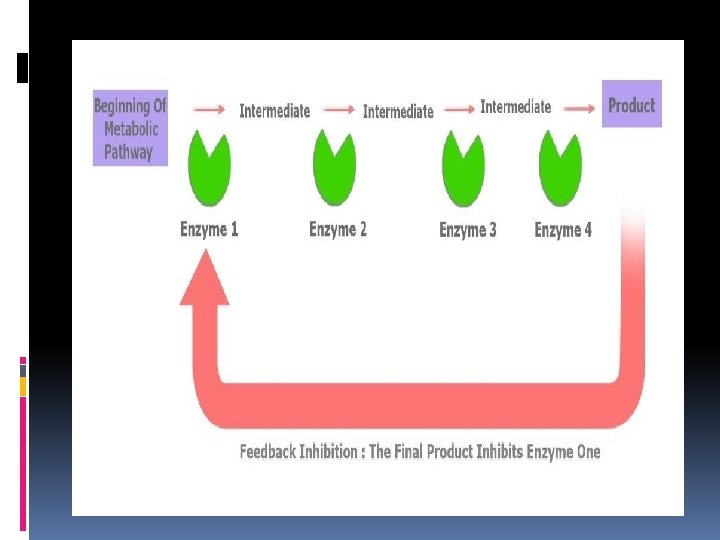
Metabolic pathway Series of reactions that takes place in a cell enzymes are used at each step in the series of reactions to make the next substrate until the desired final substrate is made Once enough of the final substrate has been made, it inhibits the enzyme at the beginning of the metabolic pathway This slows or stops production This is called a negative feedback loop
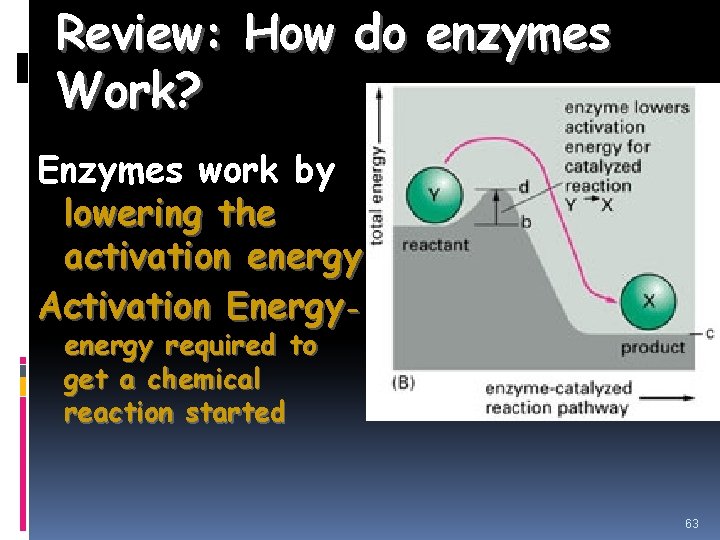
Review: How do enzymes Work? Enzymes work by lowering the activation energy. Activation Energyenergy required to get a chemical reaction started 63
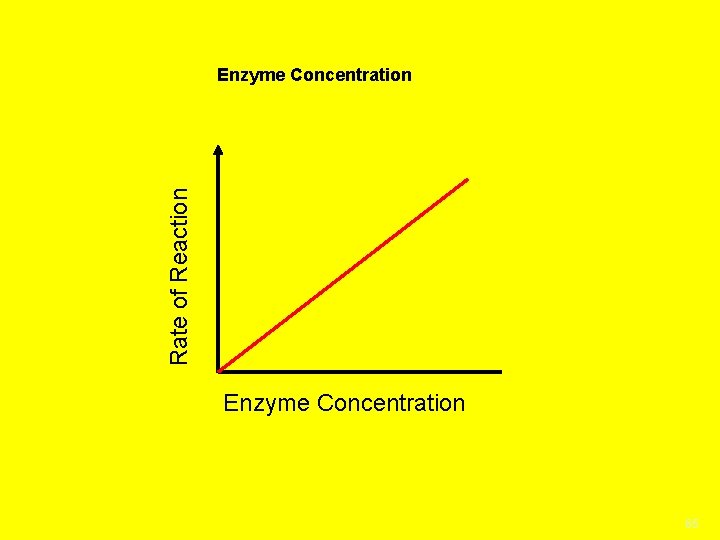
Rate of Reaction Enzyme Concentration 65
- Slides: 66