Chemistry of III B group General Trends among
Chemistry of III B group

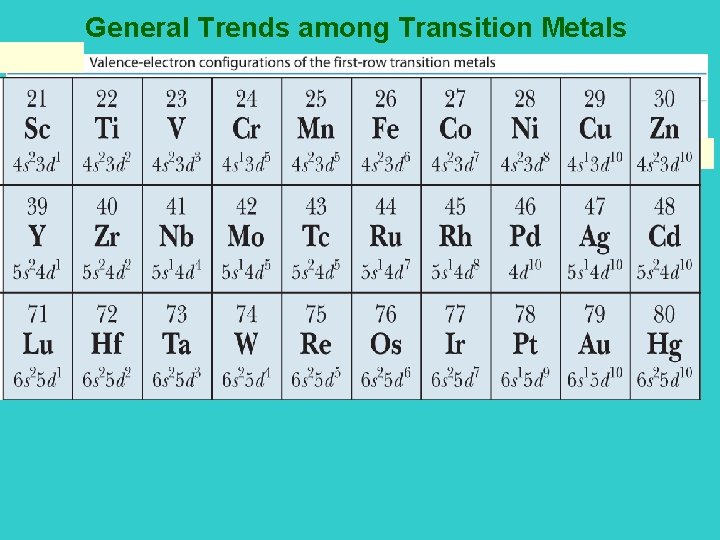
General Trends among Transition Metals
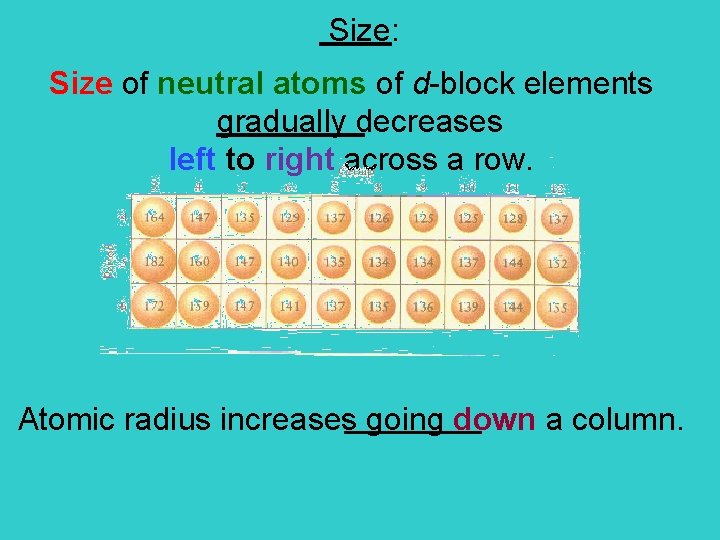
Size: Size of neutral atoms of d-block elements gradually decreases left to right across a row. Atomic radius increases going down a column.
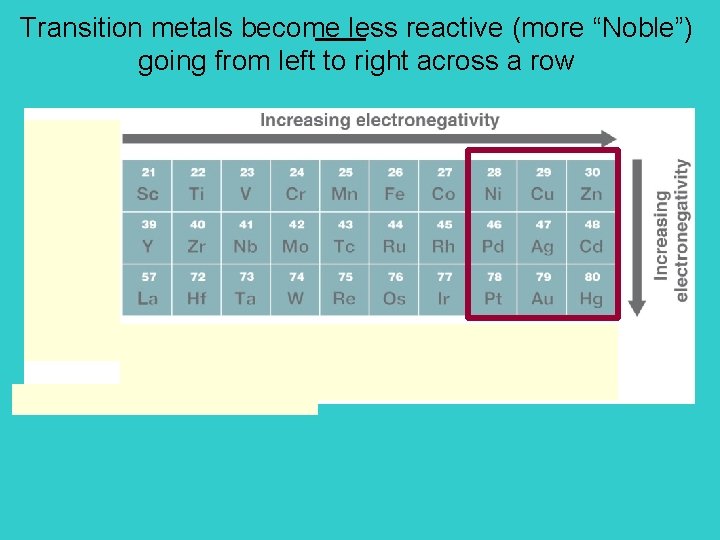
Transition metals become less reactive (more “Noble”) going from left to right across a row

Scandium

Discovered • 1878 • Lars Nilson • in the minerals euxenite and gadolinite, which had not yet been found anywhere except in Scandinavia
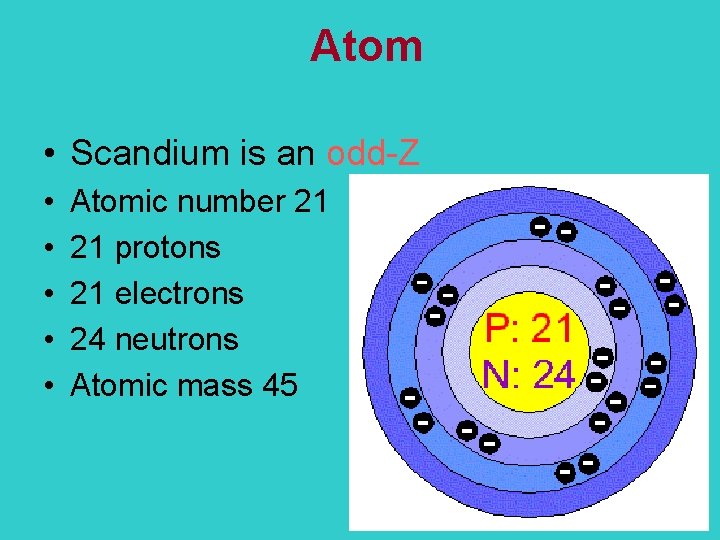
Atom • Scandium is an odd-Z • • • Atomic number 21 21 protons 21 electrons 24 neutrons Atomic mass 45

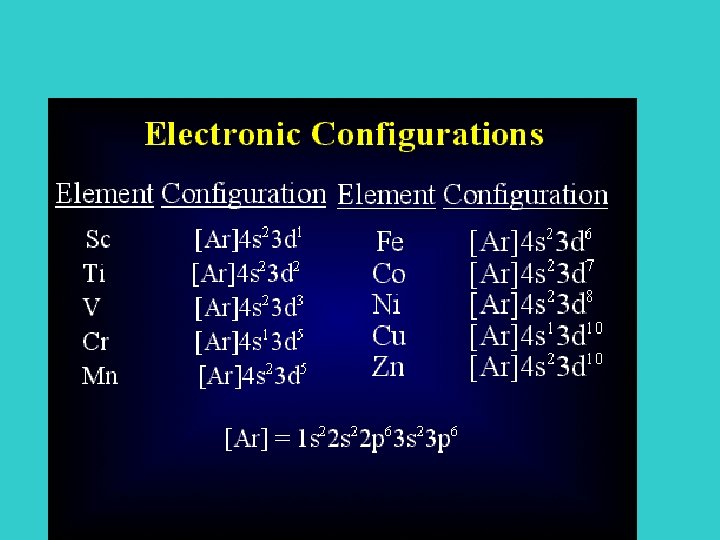
Electron Shell Configuration • • 1 s 2 2 s 22 p 6 3 s 23 p 63 d 1 4 s 2

Found • Obscure metal, 0. 0025% of earths crust. • Sc is apparently a much more abundant element in the sun and certain stars than here on earth. • It is about the 23 rd most abundant element in the sun, compared to the 50 th most abundant on earth. • It is distributed on earth, occurring in very minute quantities in over 800 mineral species. q The stable form of scandium is created in supernovas via the capture-process. Also, in addition, scandium is created by cosmic ray spallation of the more abundant iron nuclei. 28 Si + 17 n → 45 Sc (r-process) 56 Fe + p → 45 Sc + 11 C + n (cosmic ray spallation) • 45 Sc is synthesized during explosive oxygen and neon burning, and is the only stable Sc isotope.

Ores • Most scandium is presently being recovered from thortveitite • Thortveitite can contain up to 45% of scandium in the form of scandium(III) oxide. • Thortveitite ((Sc, Y)2 Si 2 O 7), • Also, is usually obtained as a by-product of refining uranium. • It is also found in the residues remaining after the extraction of tungsten from Zinnwald wolframite, • Euxenite ((Y, Ca, Er, La, Ce, U, Th)(Nb, Ta, Ti)2 O 6). • Bazzite (Be 3(Sc, Al)2 Si 6 O 18) • Wiikite

• A Pure scandium is now produced by reducing scandium fluoride with calcium metal.

Uses and applications • Approximately 20 kg (as Sc 2 O 3) of scandium is used annually in the United States to make high-intensity discharge lamps. • Scandium iodide, along with sodium iodide, when added to a modified form of mercuryvapor lamp, produces a form of metal halide lamp, an artificial light source which produce a very white light with high color rendering index that sufficiently resembles sunlight to allow good color-reproduction with TV cameras. • The aluminium-scandium alloys contain between 0. 1% and 0. 5% of scandium. They were used in the Russian military aircraft Mig 21 and Mig 29. • Scandium(III) oxide, Sc 2 O 3, scandia is a high melting white solid used in high-temperature systems (for its resistance to heat and thermal shock), electronic ceramics, and glass composition (as a helper material).

• • Stadium lights Leak Detectors Large screen TVs Scandium combined with aluminum: baseball bats, bicycle frames, and lacrosse sticks. • It is expected that scandiumaluminum alloys will be important in the manufacture of fuel cells. • Scandium is a soft, light metal that have applications in the aerospace industry. Interest to spaceship designers • scandium is too expensive for widespread use. Uses

Isotopes • 44 Sc- lives for 3. 92 hours • 45 Sc- is stable • 46 Sc- lives for 83. 81 days, • the radioactive isotope 46 Sc is used as a tracing agent in refinery crackers for crude oil, • 47 Sc- lives for 3. 34 days • 48 Sc- lives for 43. 67 hours • 49 Sc- lives for 57. 3 minutes.

Physical Properties • Named for Scandinavia. • Silver-white • Solid • Soft • Metallic • Density: 2. 2989 g/cm³ • Melting point: 1541°C • Boiling point: 2836°C • Freezing point: <2801°F

Chemical Properties 1. 2. 3. 4. 5. 6. Scandium metal is soft and has a silvery appearance. It develops a slightly yellowish or pinkish cast when exposed to air. It is not resistant to weathering and it dissolves slowly in most dilute acids. It does not react with a 1: 1 mixture of nitric acid (HNO 3) and hydrofluoric acid, HF, presumably due to the formation of an impermeable passive layer on the surface of the metal. Combines with aluminum forming alloys Ø +3 oxidation state in compounds, e. g. Sc. Cl 3, Sc 2 O 3, etc Ø most of its compounds are colorless and diamagnetic. Sc 3+ Ø most closely resembles Al 3+. • Amphoteric gelatinous hydroxide Sc(OH)3. • found in electronic devices Sc 3+

Reactions of Scandium 1 -Reactions with water • When heated or finely divided scandium will dissolve in water to form solutions containing the aquated Sc(III) and hydrogen gas. • 2 Sc(s) + 6 H 2 O(aq)→ 2 Sc 3+(aq) + 6 OH-(aq) + 3 H 2(g) 2 -Reactions with air • Scandium tarnishes in air and burns readily to form scandium(III) oxide. • 4 Sc(s) + 3 O 2(g) → 2 Sc 2 O 3(s) 3 -Reactions with halogens • Scandium is very reactive towards all of the halogens, to produce trihalides. • 2 Sc(s) + 3 F 2(g) → 2 Sc. F 3(s) • 2 Sc(s) + 3 Cl 2(g) → 2 Sc. Cl 3(s) • 2 Sc(s) + 3 Br 2(l) → 2 Sc. Br 3(s) • 2 Sc(s) + 3 I 2(s) → 2 Sc. I 3(s) 4 -Reactions with acids • Scandium readily dissolves in dilute hydrochloric acid to form solutions containing the aquated Sc(III) ion together with hydrogen gas. • 2 Sc(s) + 6 HCl(aq) → 2 Sc 3+(aq) + 6 Cl-(aq) + 3 H 2(g)

Scandium Compounds 1 -Scandium(III) oxide, Sc 2 O 3, scandia is a high melting white solid used in high-temperature systems (for its resistance to heat and thermal shock), electronic ceramics, and glass composition (as a helper material • Physical and chemical properties • Scandium(III) oxide has the M 2 O 3 structure • Scandium(III) oxide can be prepared by igniting the metal. 4 Sc(s) + 3 O 2(g) → 2 Sc 2 O 3(s) • Sc 2 O 3 is amphoteric, dissolving in acids and alkalis • It is converted into scandium(III) chloride by reaction with excess aqueous HCl or aqueous HCl/NH 4 Cl mixtures Sc 2 O 3 + 6 HCl → 2 Sc. Cl 3 + 3 H 2 O • With alkalis it forms scandate salts, for example forming K 3 Sc(OH)6 with KOH. In this, scandium shows more similarity with aluminium oxide. [2] • It is converted into the Lewis acid scandium(III) triflate by reaction with triflic acid.

Scandium Compounds 2 -Halides and pseudohalides • The halides Sc. X 3 (X = Cl, Br, I) are very soluble in water, but Sc. F 3 is insoluble. • In all four halides the scandium is 6 coordinate. • The halides are Lewis acids; for example, Sc. F 3 dissolves a solution containing excess fluoride to form [Sc. F 6]3−. The coordination number 6 is typical of Sc(III). • Scandium(III) triflate is sometimes used as a catalyst in organic chemistry.
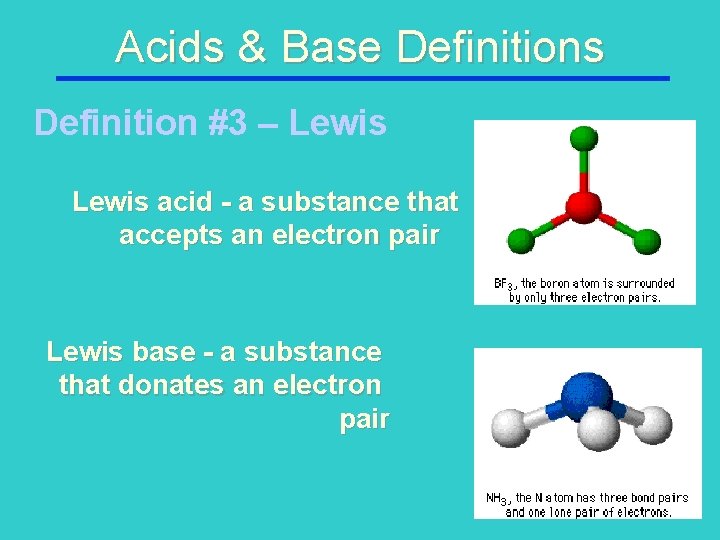
Acids & Base Definitions Definition #3 – Lewis acid - a substance that accepts an electron pair Lewis base - a substance that donates an electron pair
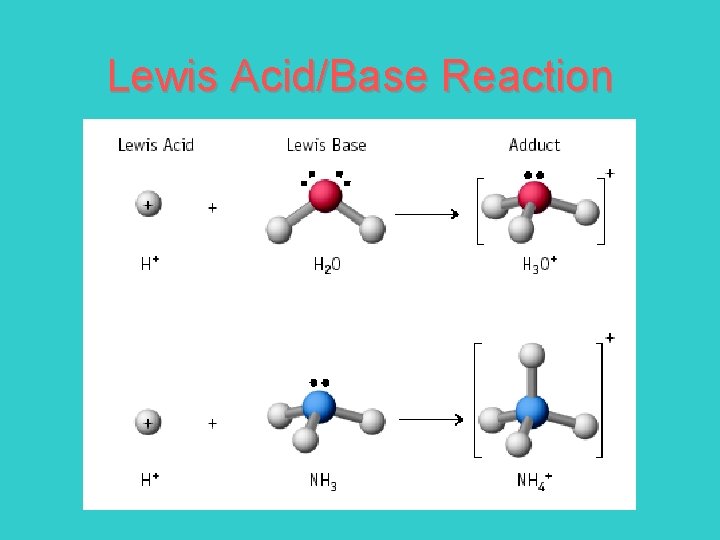
Lewis Acid/Base Reaction

Scandium(III) chloride

Scandium(III) chloride • Scandium(III) chloride, Sc. Cl 3, is a white ionic compound which is deliquescent and highly water soluble. • Scandium(III) chloride is hygroscopic it should be stored in a desiccator. • Scandium(III) chloride is offer both the anhydrous form and hexahydrate (Sc. Cl 3 • 6 H 2 O). • Sc. Cl 3 reacts with scandium metal giving a number of chlorides where scandium has an oxidation state < +3, Sc. Cl, Sc 7 Cl 10, Sc 2 Cl 3, Sc 5 Cl 8 and Sc 7 Cl 12. • Sc(s) + Sc. Cl 3 (950 °C) → Sc 5 Cl 8 • Sc(s) + Sc. Cl 3 (890 °C) → Sc 7 Cl 12 → Sc(Sc 6 Cl 12) • Reaction of Sc. Cl 3 with an alkali metal(M) and scandium metal gives the compound MSc. Cl 3 • Sc. Cl 3 has been converted to its dodecyl sulfate salt, which is used as a "Lewis acid-surfactant combined catalyst" (LASC) in aldol-like reactions • Scandium(III) chloride is found in halide lamps, optical fibers, electronic ceramics, and lasers.

Scandium(III) fluoride
• • • Scandium(III) fluoride, Sc. F 3, is an ionic compound. It is slightly soluble in water but dissolves in the presence of excess fluoride to form Sc. F 63−. Sc. F 3 can be produced by reacting scandium and fluorine. It is also formed during the extraction from the ore thortveitite by the reaction of Sc 2 O 3 with ammonium bifluoride at high temperature: Sc 2 O 3 + NH 4 HF 2 → 2 Sc. F 3 + 6 NH 4 F + 3 H 2 O The resulting mixture contains a number of metal fluorides and this is reduced by reaction with calcium metal at high temperature
Scandium(III) trifluoromethanesulfonate
Scandium(III) trifluoromethanesulfonate • Scandium triflate or Sc(OTf)3 is a chemical compound composed of scandium with triflate counterions. • Scandium triflate is used as a reagent in organic chemistry as a Lewis acid. Compared to other Lewis acids this reagent is stable towards water and can often be used in an organic reaction as a true catalyst rather than one used in stoichiometric amounts. • The compound is prepared by reaction of scandium oxide with triflic acid. • An example of the use of scandium triflate is the Mukaiyama aldol addition reaction between benzaldehyde and the silyl enol ether of cyclohexanone with an 81% chemical yield.

Scandium(III) sulfide

Scandium(III) sulfide • Scandium(III) sulfide with the chemical formula Sc 2 S 3. • The crystal structure of Sc 2 S 3 is closely related to that of sodium chloride, • The normal way to make metal sulfides is simply to mix the two elements and heat them, but in the case of scandium, this method yields scandium monosulfide, Sc. S. • Sc 2 S 3 can be prepared by heating scandium(III) oxide and hydrogen sulfide in a graphite crucible to 1550 °C or above for 2– 3 hours. • Sc 2 O 3 + 3 H 2 S → Sc 2 S 3 + 3 H 2 O • Above 1100 °C, Sc 2 S 3 loses sulfur, forming nonstoichiometric compounds such as Sc 1. 37 S 2.
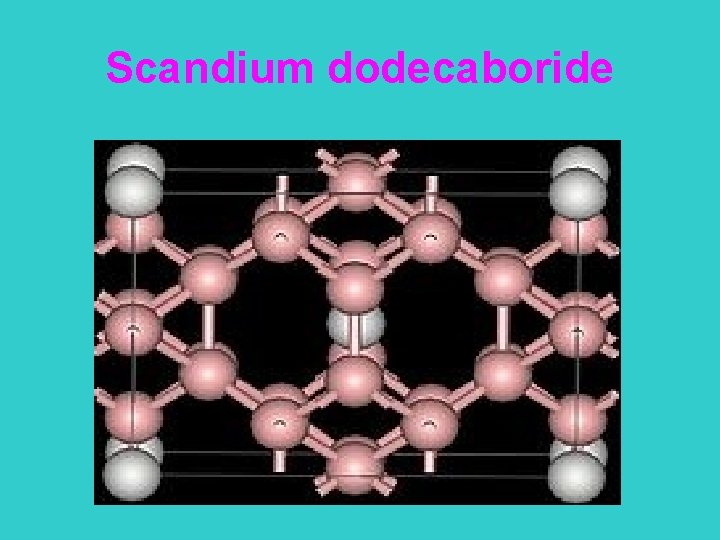
Scandium dodecaboride

Scandium dodecaboride as Sc. B 12 • Scandium dodecaboride is a refractory metal boride • Sc. B 12 is formed by mixing a 7: 1 ratio of boron powder and scandium oxide powder, heating to 2500 °C

Health and safety • Elemental scandium is not considered to be toxic. • Little animal testing of scandium compounds has been done. • The median lethal dose (LD 50) levels for scandium(III) chloride for rats have been determined and were intraperitoneal 4 mg/kg and oral 755 mg/kg • In the light of these results compounds of scandium should be handled as compounds of moderate toxicity.
- Slides: 35