Chemistry of Cells Has nothing to do with

















- Slides: 45

Chemistry of Cells

Has nothing to do with being naturally occurring!!

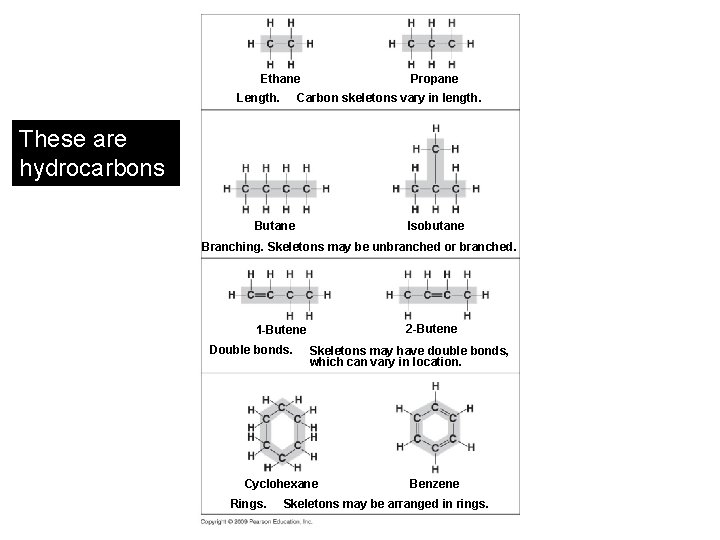
Propane Ethane Length. Carbon skeletons vary in length. These are hydrocarbons Isobutane Branching. Skeletons may be unbranched or branched. 2 -Butene 1 -Butene Double bonds. Skeletons may have double bonds, which can vary in location. Cyclohexane Rings. Benzene Skeletons may be arranged in rings.

Study molecules important to life 4 Main Groups

What elements do they contain? ? ? Carbon, Hydrogen, and Oxygen


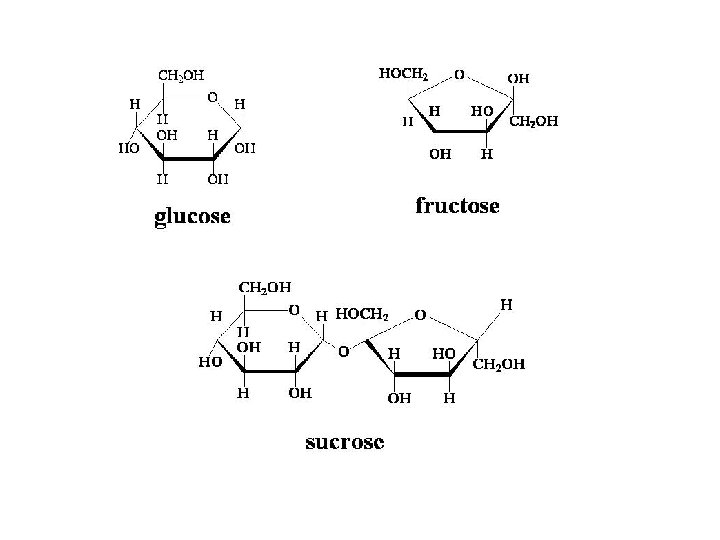
1) Monosaccharides (Simple sugars) Examples: -glucose BLOOD SUGAR -fructose FOUND IN FRUITS -Ribose found in RNA Function energy (readily available)

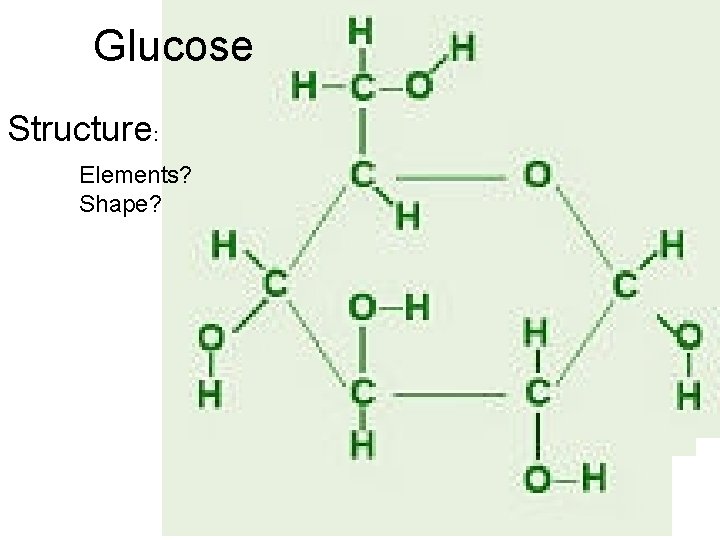
Glucose Structure: Elements? Shape?


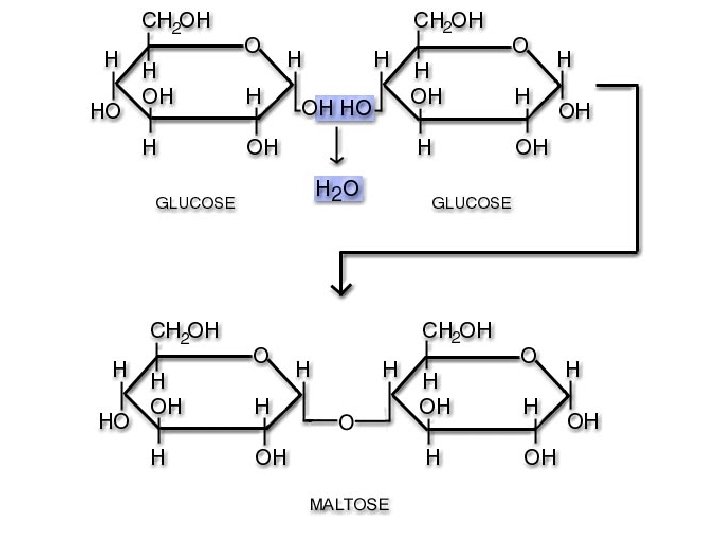
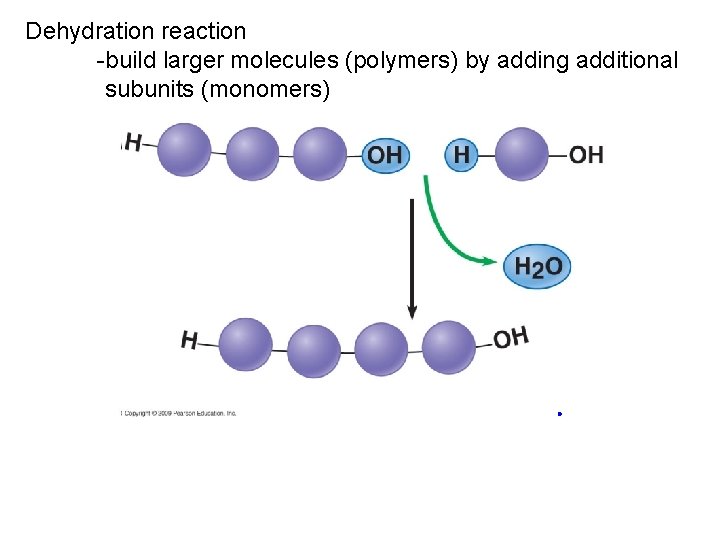
Dehydration reaction -build larger molecules (polymers) by adding additional subunits (monomers)

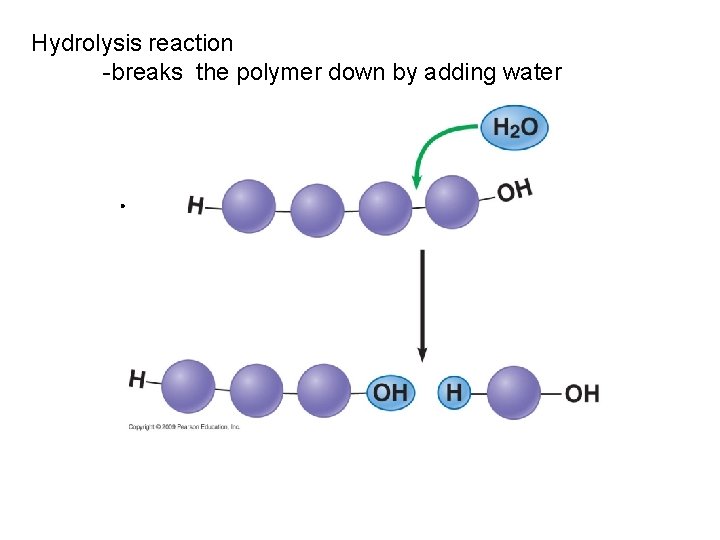
Hydrolysis reaction -breaks the polymer down by adding water

Disaccharides • Examples: – Sucrose – Lactose – Maltose Structure: formed from the joining of two monosaccharides • Functions - Energy Maltose


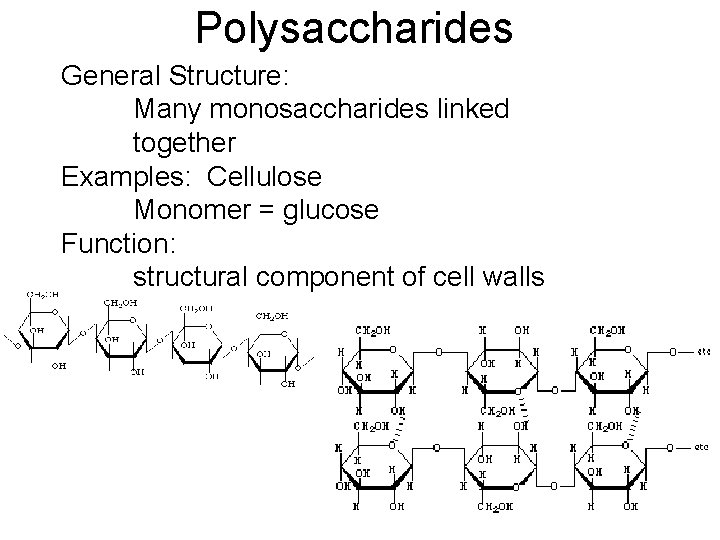
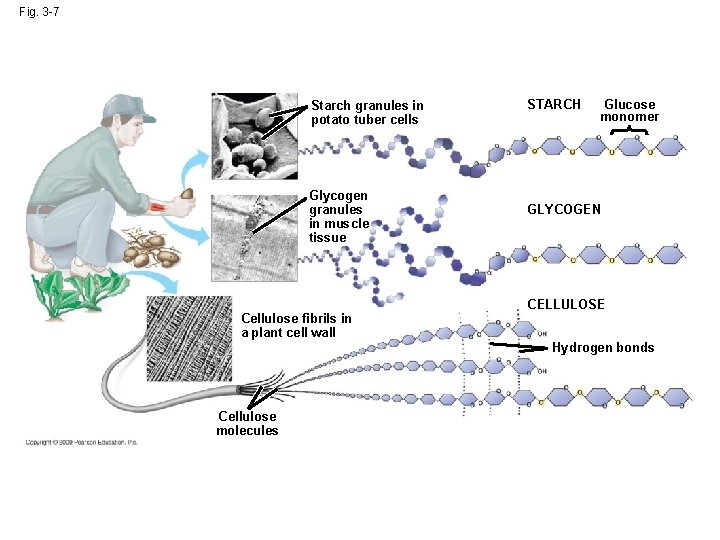
Polysaccharides General Structure: Many monosaccharides linked together Examples: Cellulose Monomer = glucose Function: structural component of cell walls

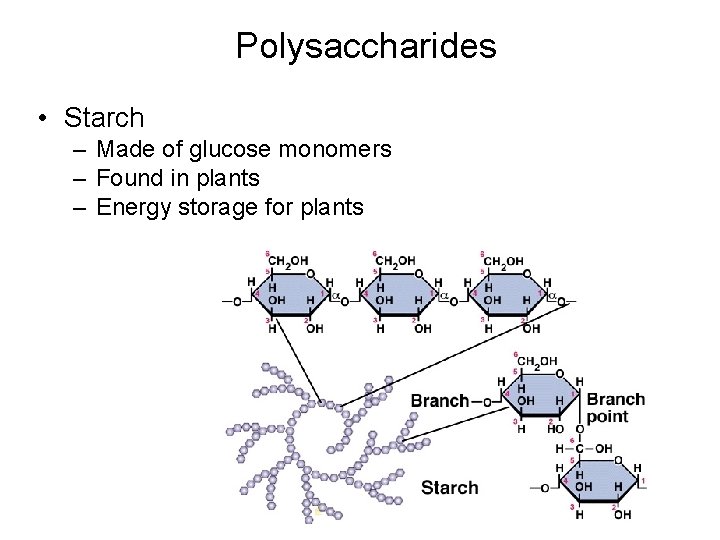
Polysaccharides • Starch – Made of glucose monomers – Found in plants – Energy storage for plants

Glycogen • Glucose monomers • How animals store glucose – In liver and muscles

Fig. 3 -7 Starch granules in potato tuber cells Glycogen granules in muscle tissue STARCH Glucose monomer GLYCOGEN CELLULOSE Cellulose fibrils in a plant cell wall Hydrogen bonds Cellulose molecules

Chitin • Found in the exoskeletons of arthropods • Function? ?

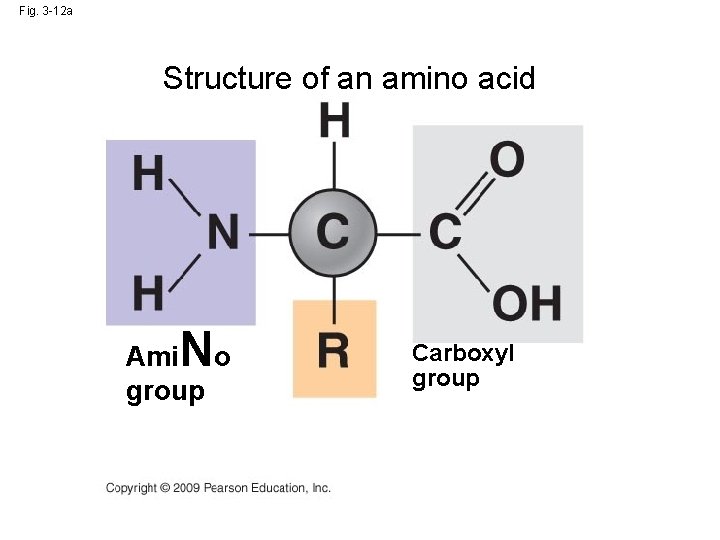
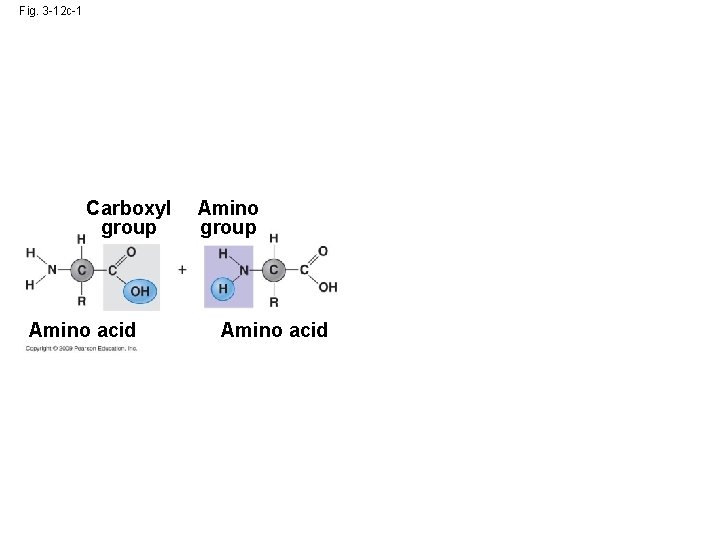
Proteins structure • Are polymers • Made from chains of amino acids • Linked by peptide bonds – Those bonds form through dehydration reaction

Fig. 3 -12 a Structure of an amino acid N Ami o group Carboxyl group

Fig. 3 -12 c-1 Carboxyl group Amino acid Amino group Amino acid

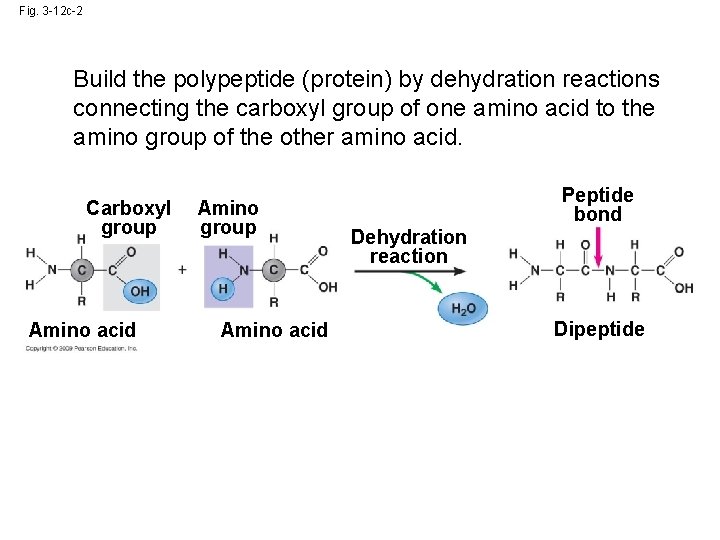
Fig. 3 -12 c-2 Build the polypeptide (protein) by dehydration reactions connecting the carboxyl group of one amino acid to the amino group of the other amino acid. Carboxyl group Amino acid Amino group Amino acid Peptide bond Dehydration reaction Dipeptide

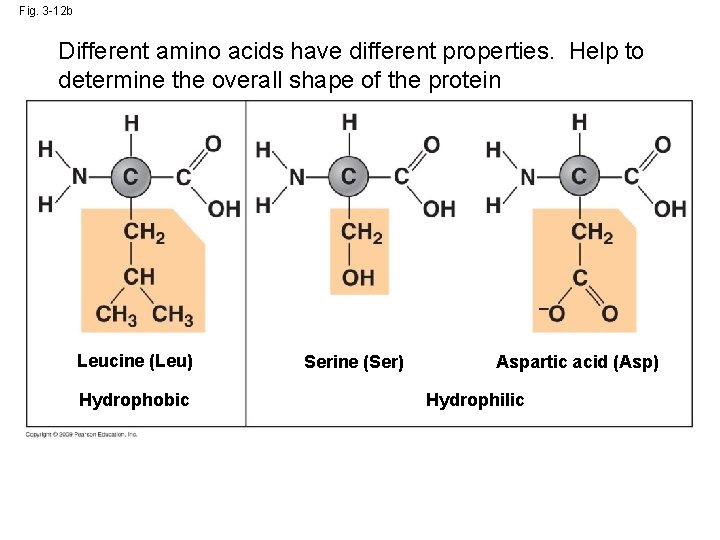
Fig. 3 -12 b Different amino acids have different properties. Help to determine the overall shape of the protein Leucine (Leu) Hydrophobic Serine (Ser) Aspartic acid (Asp) Hydrophilic

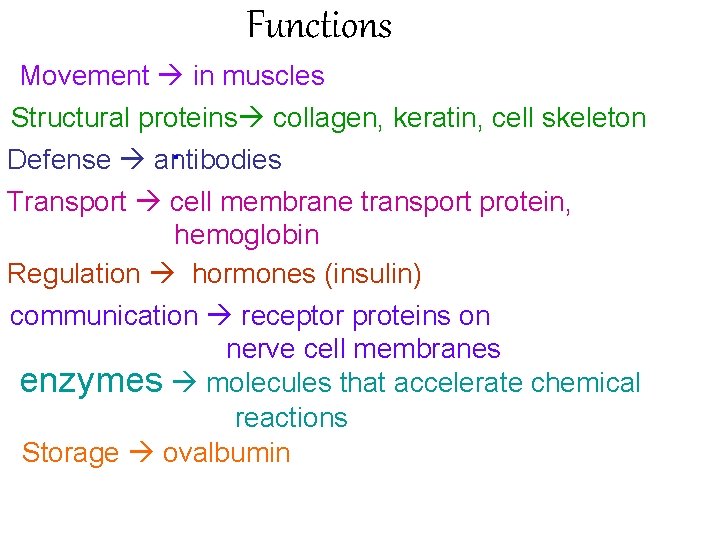
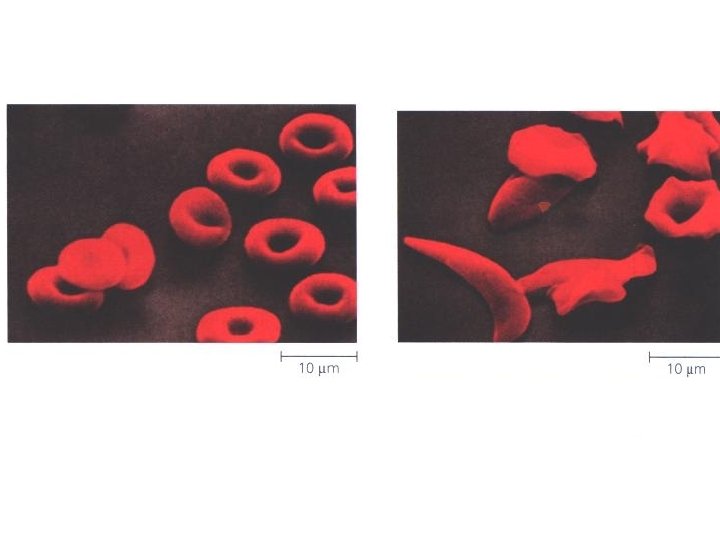
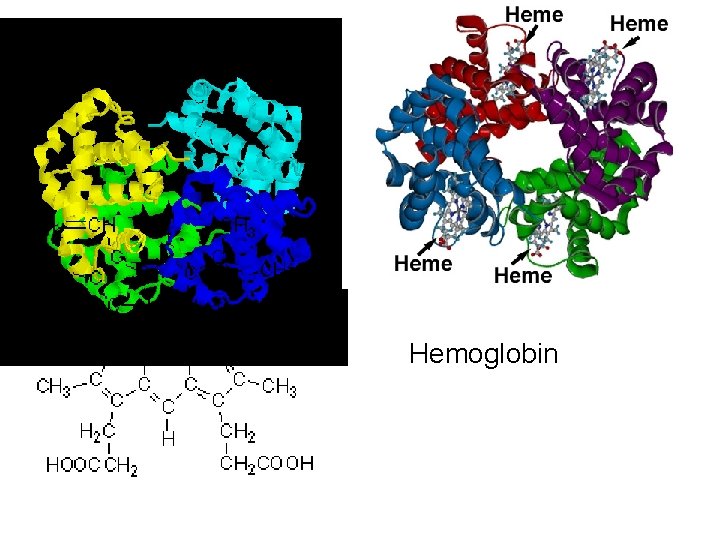
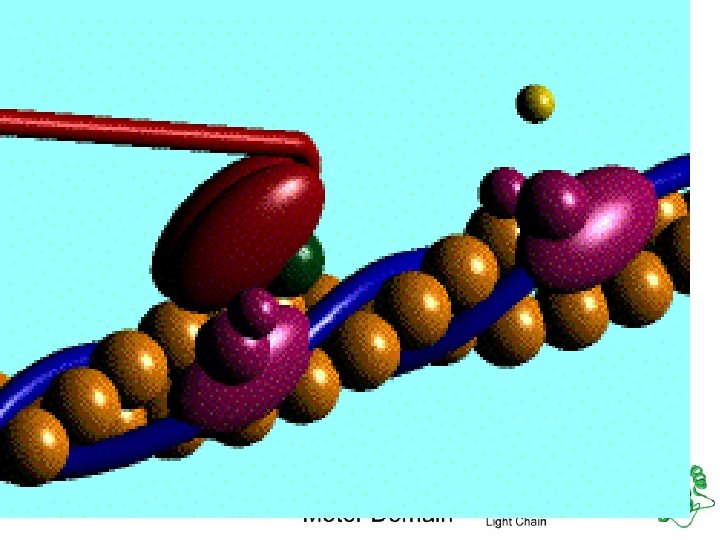
Functions Movement in muscles Structural proteins collagen, keratin, cell skeleton Defense antibodies Transport cell membrane transport protein, hemoglobin Regulation hormones (insulin) communication receptor proteins on nerve cell membranes enzymes molecules that accelerate chemical reactions Storage ovalbumin




Hemoglobin

Insulin collagen

actin myosin

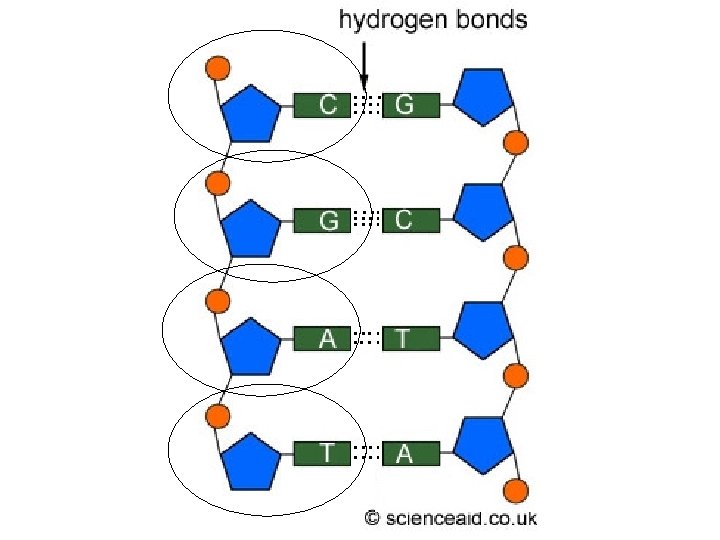
Nucleic Acids • Are polymers made of linked nucleotides • Examples are DNA and RNA


Fig. 3 -8 a

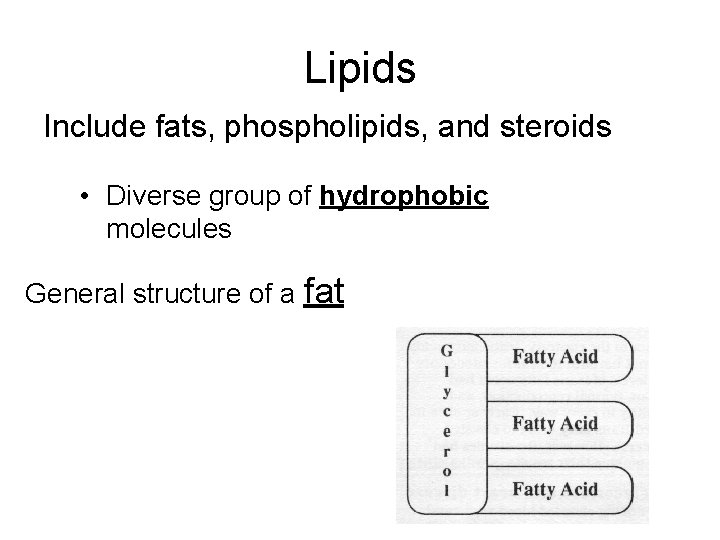
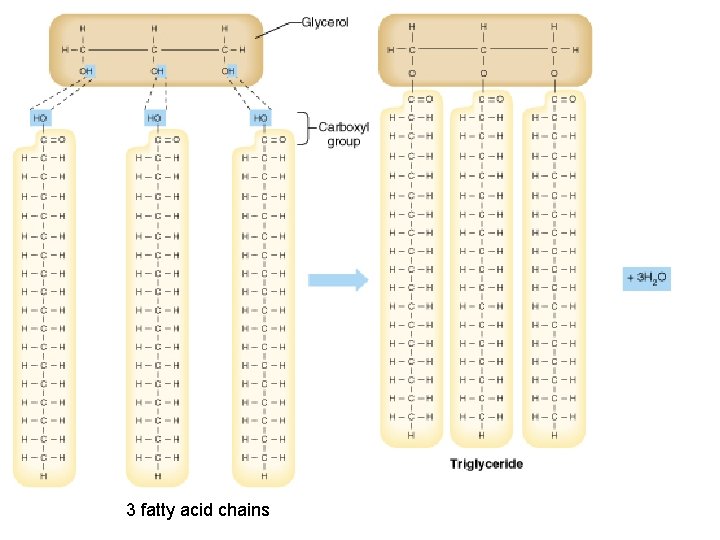
Lipids Include fats, phospholipids, and steroids • Diverse group of hydrophobic molecules General structure of a fat

3 fatty acid chains

Fig. 3 -8 b Built by dehydration reactions Glycerol Fatty acid


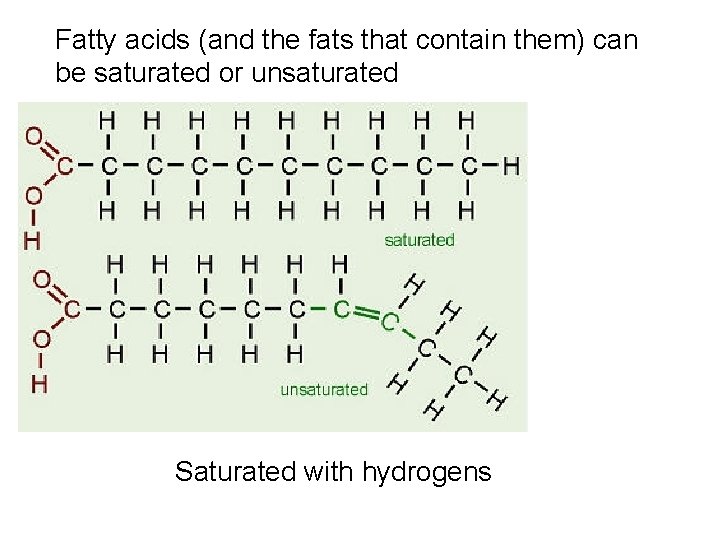
Fatty acids (and the fats that contain them) can be saturated or unsaturated Saturated with hydrogens

Amount of Hydrogens Saturated Unsaturated Shape Presence of of tails double bonds Solid or liquid (room temp) Source/ examples

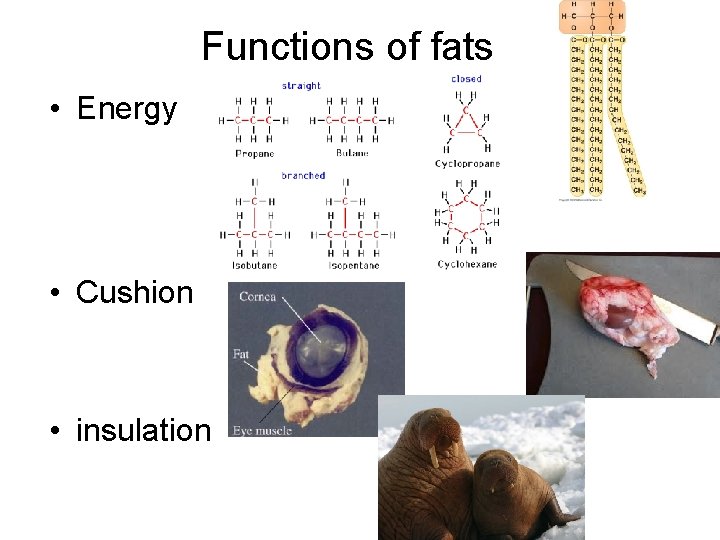
Functions of fats • Energy • Cushion • insulation

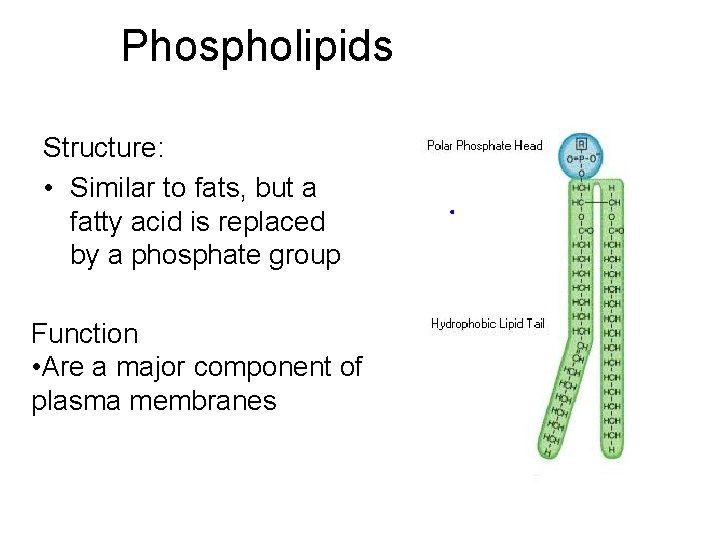
Phospholipids Structure: • Similar to fats, but a fatty acid is replaced by a phosphate group Function • Are a major component of plasma membranes

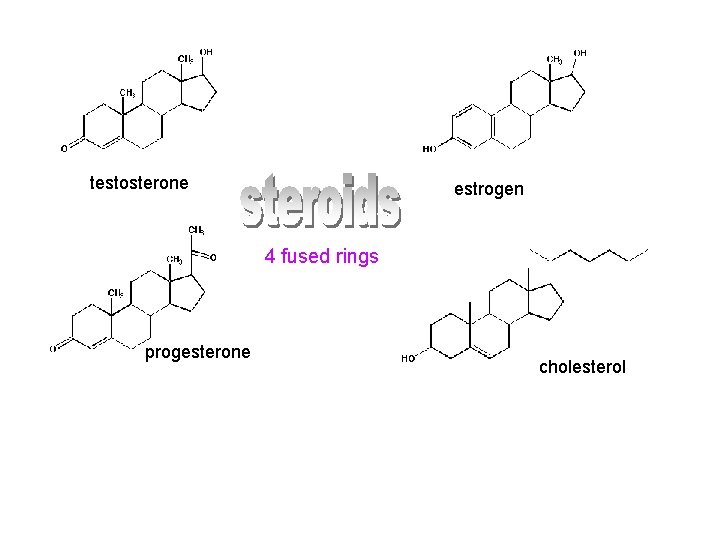
testosterone estrogen 4 fused rings progesterone cholesterol

Study molecules important to life 4 Main Groups
 Nothing but nothing leaves nothing
Nothing but nothing leaves nothing The way my mother speaks carol ann duffy annotated
The way my mother speaks carol ann duffy annotated Alesha the boy does nothing
Alesha the boy does nothing Etymology and morphology
Etymology and morphology Nothing venture nothing have
Nothing venture nothing have Waters view with open mouth
Waters view with open mouth Regulation of tubular reabsorption
Regulation of tubular reabsorption Pineal gland
Pineal gland Somatic vs gamete
Somatic vs gamete Somatic cells vs germ cells
Somatic cells vs germ cells Red blood cells and white blood cells difference
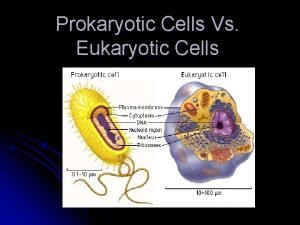
Red blood cells and white blood cells difference Prokaryotic v. eukaryotic cells
Prokaryotic v. eukaryotic cells Animal rights and animal welfare venn diagram
Animal rights and animal welfare venn diagram Prokaryotes vs eukaryotes venn diagram
Prokaryotes vs eukaryotes venn diagram Why did robert hooke name cells “cells”?
Why did robert hooke name cells “cells”? Masses of cells form and steal nutrients from healthy cells
Masses of cells form and steal nutrients from healthy cells Label
Label Are plant cells prokaryotic or eukaryotic
Are plant cells prokaryotic or eukaryotic Which compares prokaryotes and eukaryotes
Which compares prokaryotes and eukaryotes Chapter 8 cellular reproduction cells from cells
Chapter 8 cellular reproduction cells from cells Cells and life lesson 1 answer key
Cells and life lesson 1 answer key Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Mitosis and meiosis
Mitosis and meiosis Frank has an eraser it has a mass of 4g
Frank has an eraser it has a mass of 4g A problem has been detected and windows shut down
A problem has been detected and windows shut down Windows shut down to prevent damage
Windows shut down to prevent damage Spring has sprung, fall has fell poem
Spring has sprung, fall has fell poem Every picture has a story and every story has a moment
Every picture has a story and every story has a moment Whoever has ears let them hear
Whoever has ears let them hear You mustn't try to build up a wall analysis
You mustn't try to build up a wall analysis Assertive sentence
Assertive sentence The meaning of the poem nothing gold can stay
The meaning of the poem nothing gold can stay Much ado about nothing allusions
Much ado about nothing allusions Storm on the island heaney
Storm on the island heaney My mistress' eyes are nothing like the sun 해석
My mistress' eyes are nothing like the sun 해석 Saturday climbing questions and answers
Saturday climbing questions and answers Nothing happens twice
Nothing happens twice Nothing matters question tag
Nothing matters question tag Poetry makes nothing happen analysis
Poetry makes nothing happen analysis Neighbor
Neighbor Nothings changes
Nothings changes Expand gobi fff campaign
Expand gobi fff campaign Nothing is more precious than happiness and health
Nothing is more precious than happiness and health What is the figurative language in nothing gold can stay
What is the figurative language in nothing gold can stay So eden sank to grief meaning
So eden sank to grief meaning