Chemistry of Cells Chapter 2 Section 3 Dr



- Slides: 14

Chemistry of Cells Chapter 2, Section 3 Dr. Steve W. Altstiel Naples High School

Objectives: • Summarize the characteristics of organic compounds. • Compare the structure and function of different types of biomolecules. • Describe the components of DNA and RNA. • State the main role of ATP in cells.

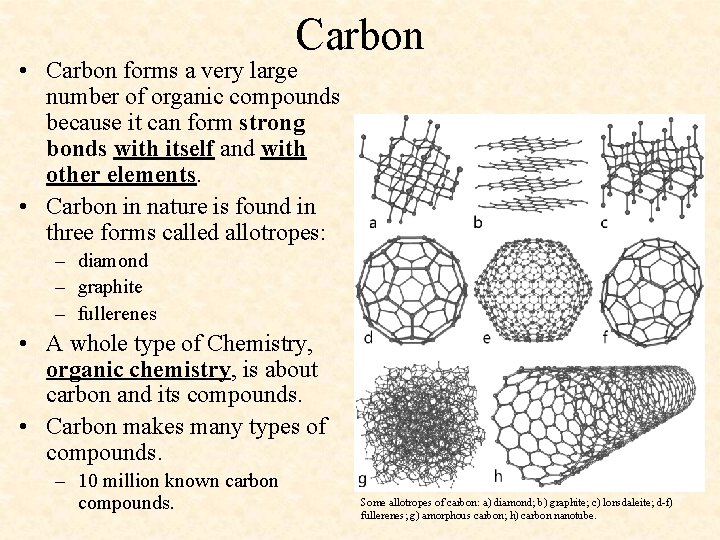
Carbon • Carbon forms a very large number of organic compounds because it can form strong bonds with itself and with other elements. • Carbon in nature is found in three forms called allotropes: – diamond – graphite – fullerenes • A whole type of Chemistry, organic chemistry, is about carbon and its compounds. • Carbon makes many types of compounds. – 10 million known carbon compounds. Some allotropes of carbon: a) diamond; b) graphite; c) lonsdaleite; d-f) fullerenes; g) amorphous carbon; h) carbon nanotube.


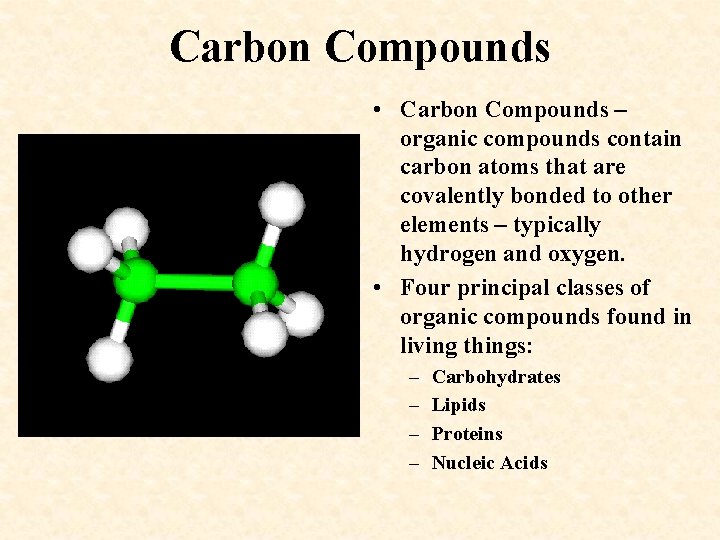
Carbon Compounds • Carbon Compounds – organic compounds contain carbon atoms that are covalently bonded to other elements – typically hydrogen and oxygen. • Four principal classes of organic compounds found in living things: – – Carbohydrates Lipids Proteins Nucleic Acids

Carbohydrates – organic compounds made of carbon, hydrogen, and oxygen. (1: 2: 1 ratio) • Key source of energy. • Found in most foods, especially fruits, vegetables, and grains.

Carbohydrates Cont. . • Monosaccharides – building blocks of carbohydrates. – Glucose, C 6 H 12 O 6 – Major source of energy. – Fructose • Disaccharides – double sugars formed when two monosaccharides are joined. – Glucose + Fructose = Sucrose (table sugar)

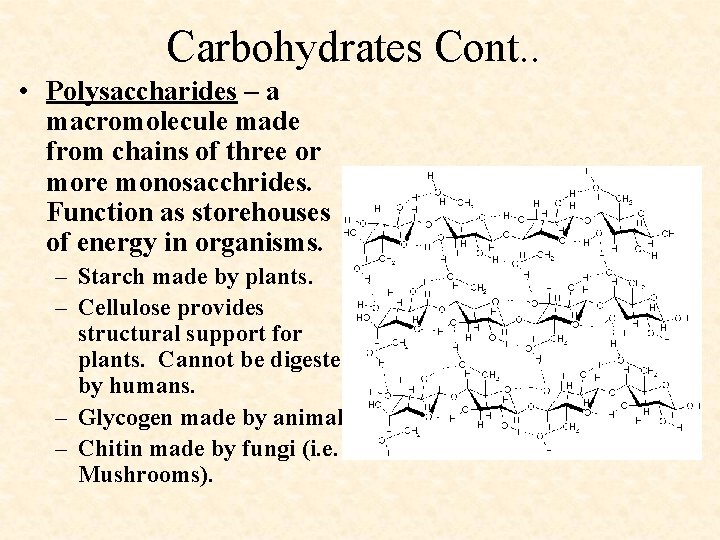
Carbohydrates Cont. . • Polysaccharides – a macromolecule made from chains of three or more monosacchrides. Function as storehouses of energy in organisms. – Starch made by plants. – Cellulose provides structural support for plants. Cannot be digested by humans. – Glycogen made by animals. – Chitin made by fungi (i. e. Mushrooms).

Lipids • Lipids – Nonpolar molecules that are not soluble or mostly insoluble in water. Fats, phospholipids, steroids, and waxes.

Proteins • Proteins – usually a large molecule formed by linked smaller molecules called amino acids.

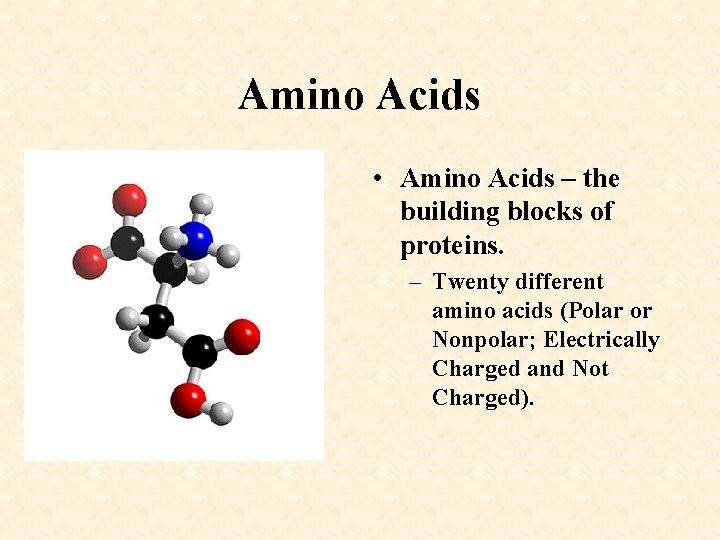
Amino Acids • Amino Acids – the building blocks of proteins. – Twenty different amino acids (Polar or Nonpolar; Electrically Charged and Not Charged).

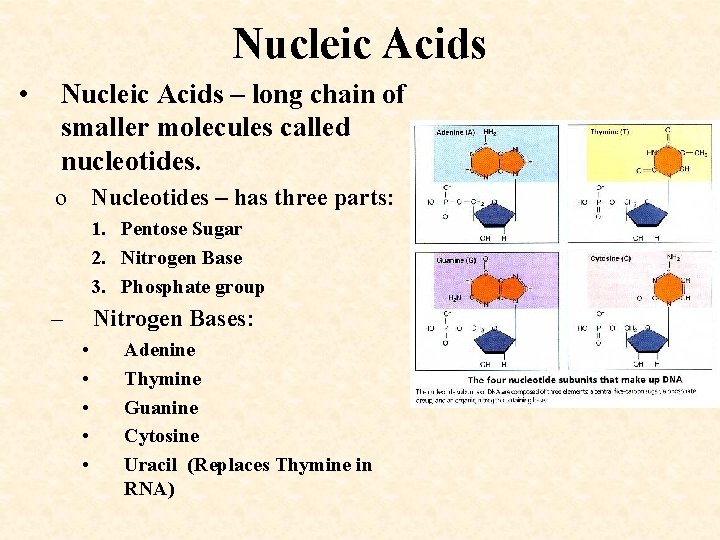
Nucleic Acids • Nucleic Acids – long chain of smaller molecules called nucleotides. o Nucleotides – has three parts: 1. Pentose Sugar 2. Nitrogen Base 3. Phosphate group – Nitrogen Bases: • • • Adenine Thymine Guanine Cytosine Uracil (Replaces Thymine in RNA)

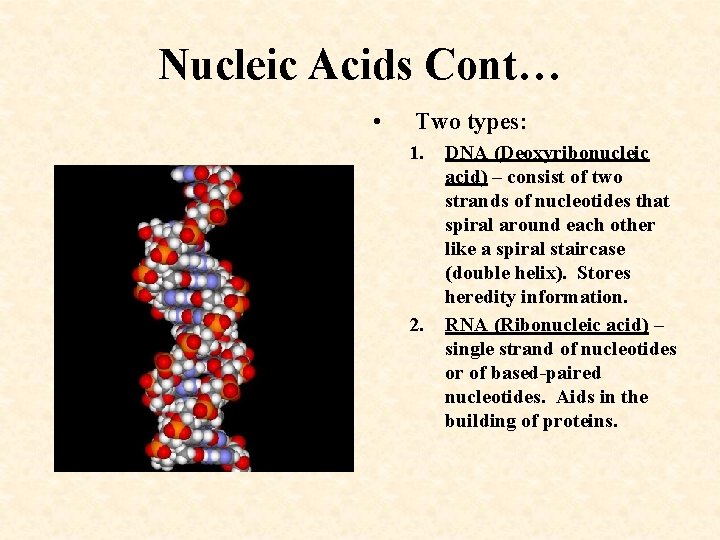
Nucleic Acids Cont… • Two types: 1. 2. DNA (Deoxyribonucleic acid) – consist of two strands of nucleotides that spiral around each other like a spiral staircase (double helix). Stores heredity information. RNA (Ribonucleic acid) – single strand of nucleotides or of based-paired nucleotides. Aids in the building of proteins.

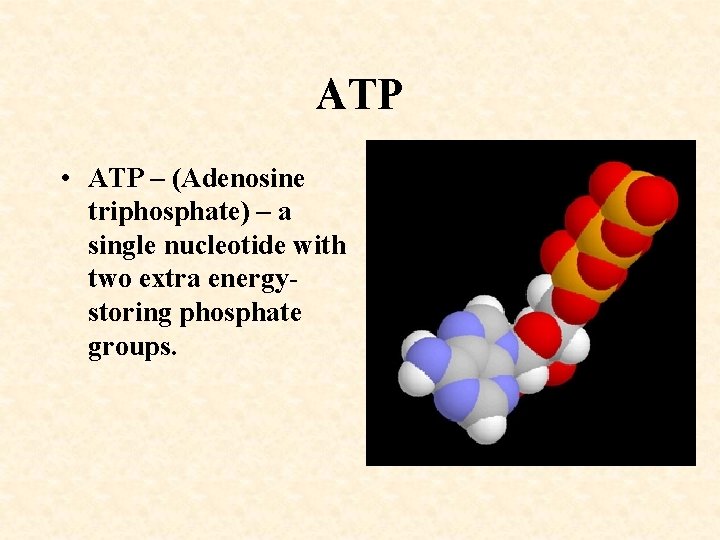
ATP • ATP – (Adenosine triphosphate) – a single nucleotide with two extra energystoring phosphate groups.