Chemistry of Carbon Building Blocks of Life Why
Chemistry of Carbon Building Blocks of Life
Why do we study carbon? All of life is built on carbon Cells… ~72% H 2 O ~25% carbon compounds carbohydrates lipids proteins nucleic acids ~3% salts Na, Cl, K… What is the main source of carbon? CO 2
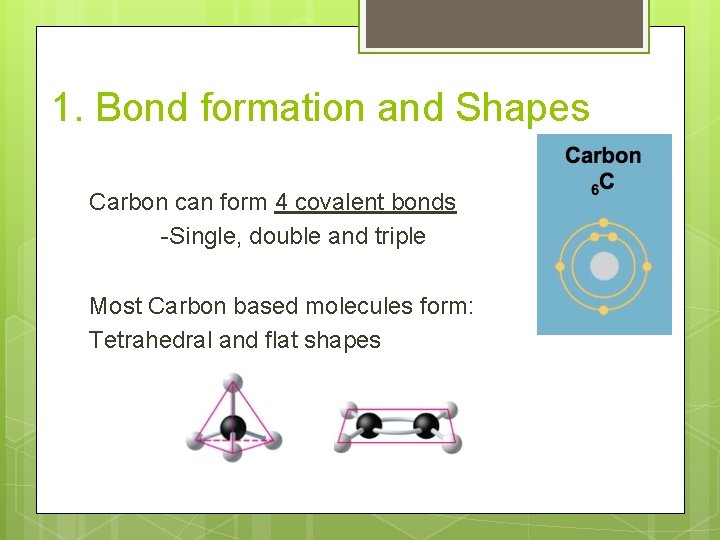
1. Bond formation and Shapes Carbon can form 4 covalent bonds -Single, double and triple Most Carbon based molecules form: Tetrahedral and flat shapes
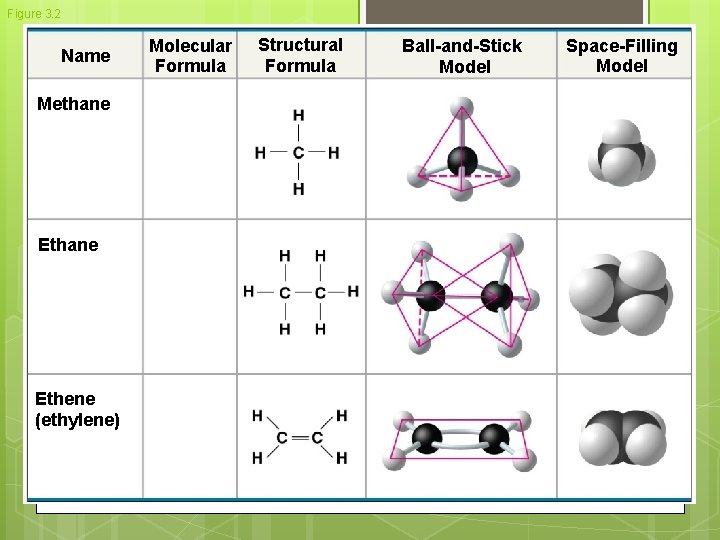
Figure 3. 2 Name Methane Ethene (ethylene) Molecular Formula Structural Formula Ball-and-Stick Model Space-Filling Model
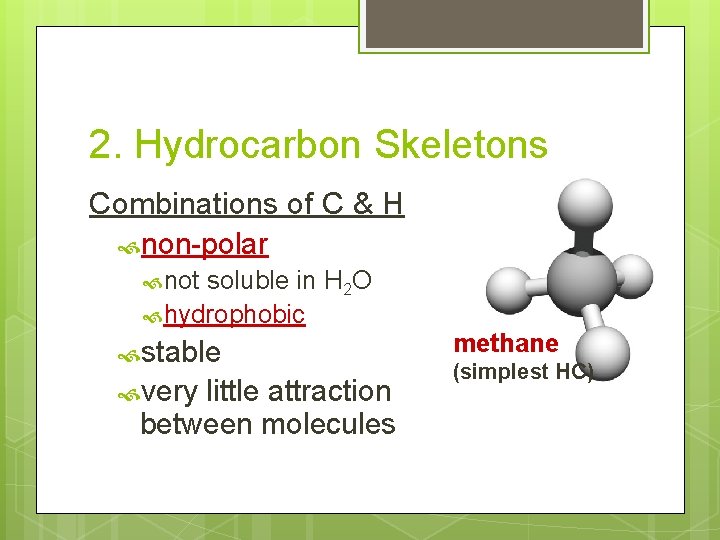
2. Hydrocarbon Skeletons Combinations of C & H non-polar not soluble in H 2 O hydrophobic stable very little attraction between molecules methane (simplest HC)
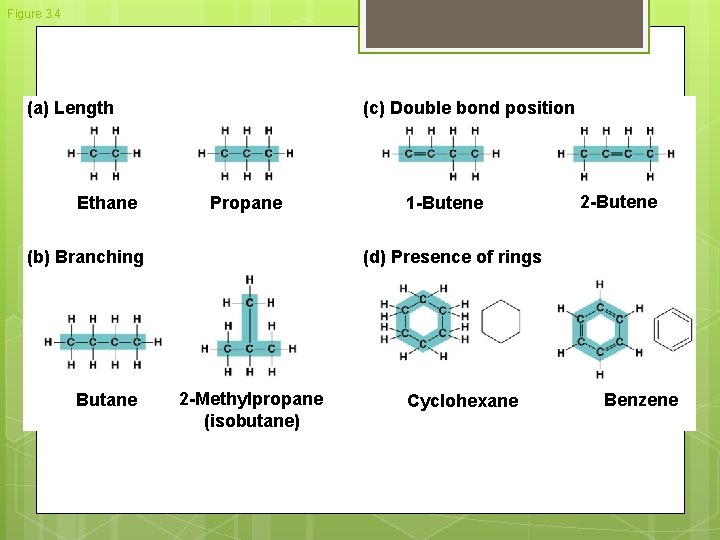
Figure 3. 4 (a) Length Ethane (c) Double bond position Propane (b) Branching Butane 1 -Butene 2 -Butene (d) Presence of rings 2 -Methylpropane (isobutane) Cyclohexane Benzene
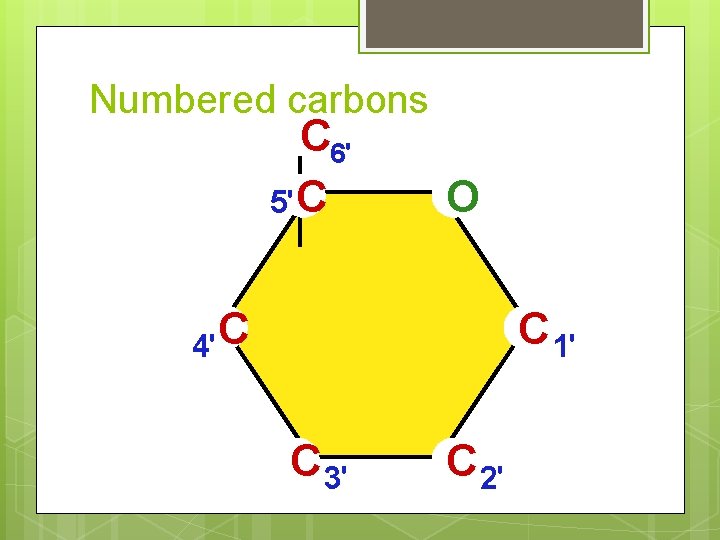
Numbered carbons C 6' 5'C O C 4' C 1' C 3' C 2'
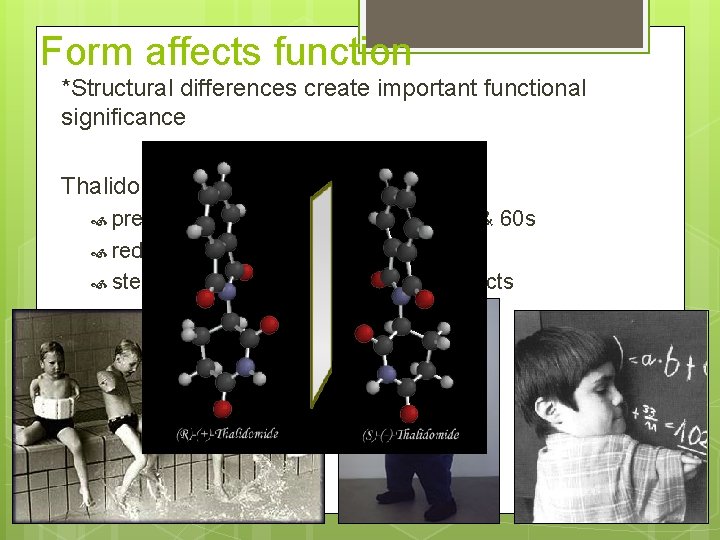
Form affects function *Structural differences create important functional significance Thalidomide prescribed to pregnant women in 50 s & 60 s reduced morning sickness, but… stereoisomer caused severe birth defects
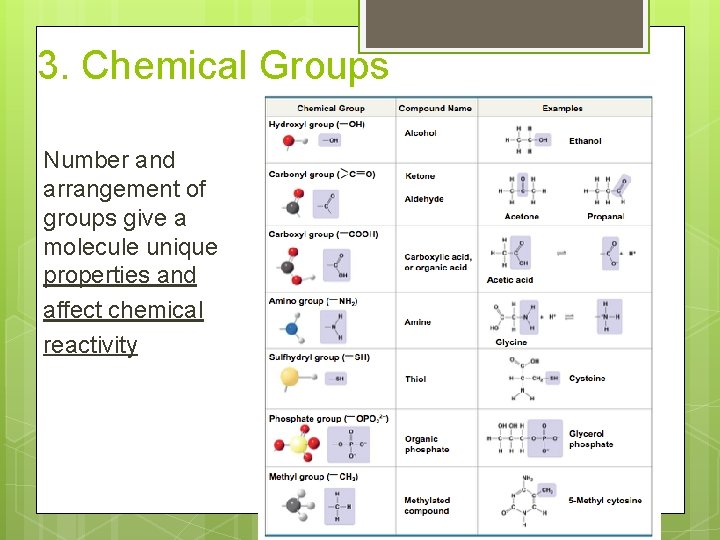
3. Chemical Groups Number and arrangement of groups give a molecule unique properties and affect chemical reactivity
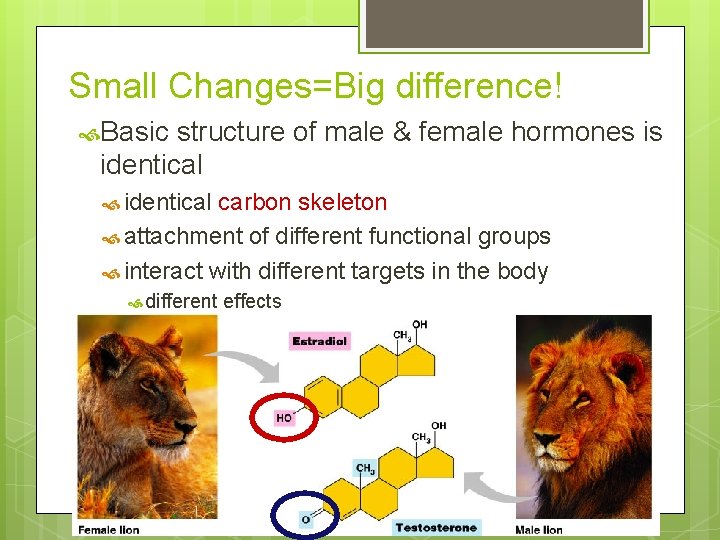
Small Changes=Big difference! Basic structure of male & female hormones is identical carbon skeleton attachment of different functional groups interact with different targets in the body different effects
4. Macromolecules Smaller carbon based molecules join together to form larger molecules macromolecules 4 major classes of macromolecules: carbohydrates lipids proteins nucleic acids
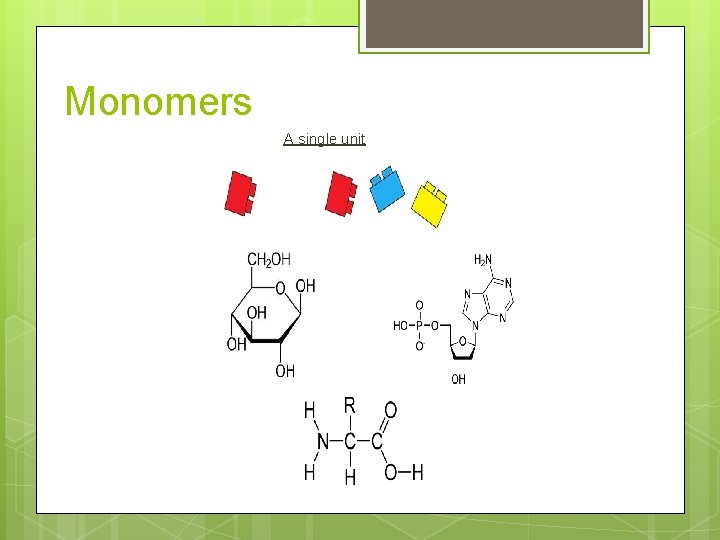
Monomers A single unit

polymers A chain of covalently bonded monomers
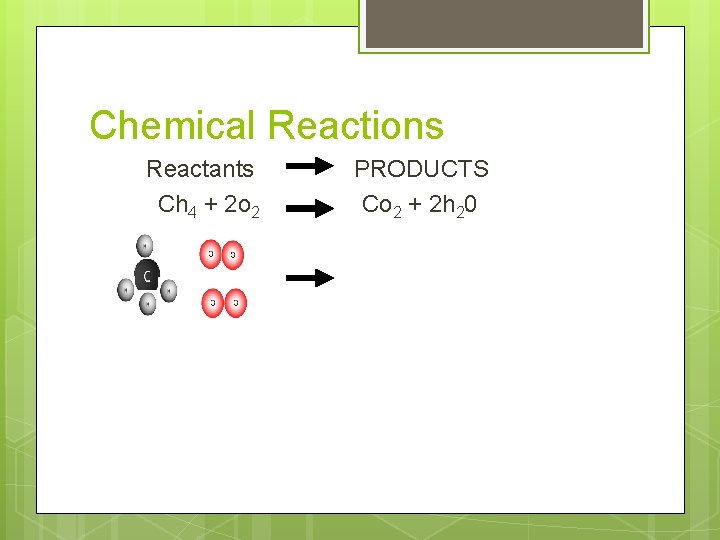
Chemical Reactions Reactants Ch 4 + 2 o 2 PRODUCTS Co 2 + 2 h 20
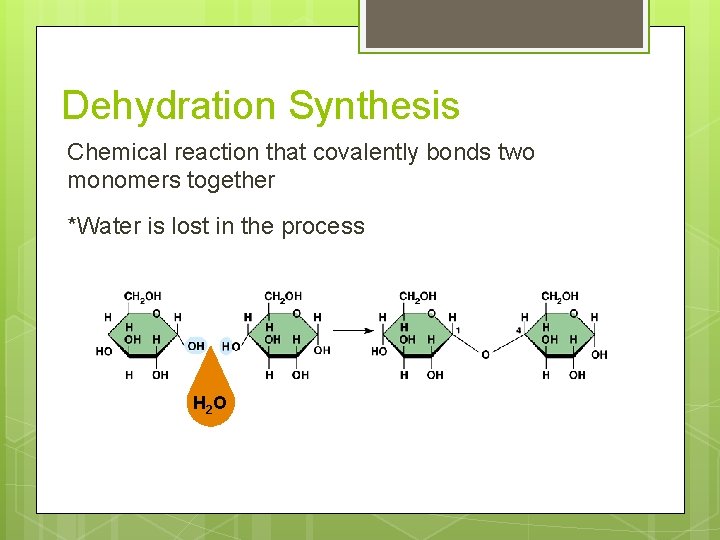
Dehydration Synthesis Chemical reaction that covalently bonds two monomers together *Water is lost in the process H 2 O
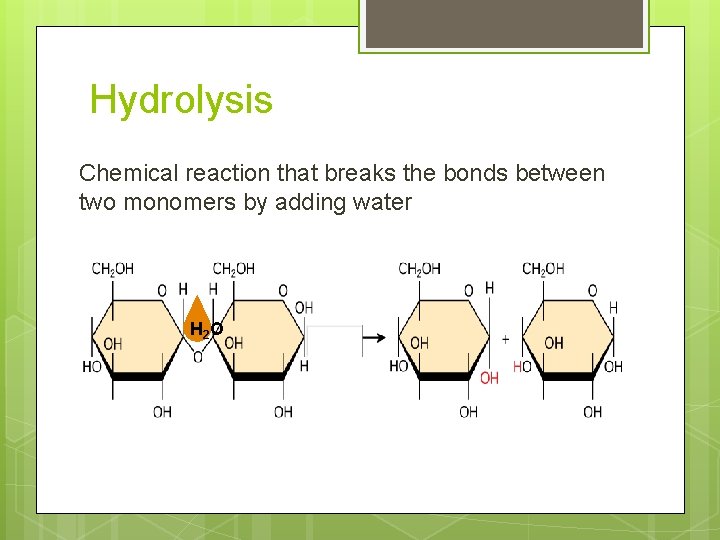
Hydrolysis Chemical reaction that breaks the bonds between two monomers by adding water H 2 O
- Slides: 16