Chemistry November 25 Types of Chemical Reactions Combination

Chemistry November 25
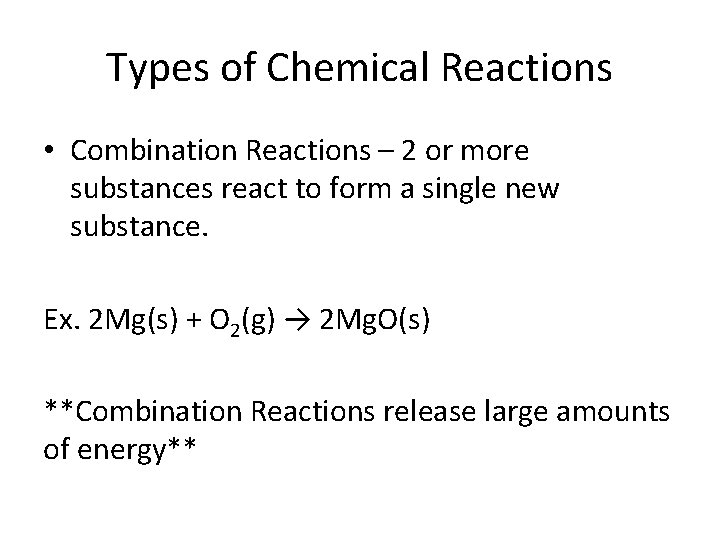
Types of Chemical Reactions • Combination Reactions – 2 or more substances react to form a single new substance. Ex. 2 Mg(s) + O 2(g) → 2 Mg. O(s) **Combination Reactions release large amounts of energy**

When 2 nonmetals react, more than one combination is often possible. Ex. S(s) + O 2(g) → SO 2(g) sulfur dioxide OR 2 S(s) + 3 O 2(g) → 2 SO 3(g) sulfur trioxide
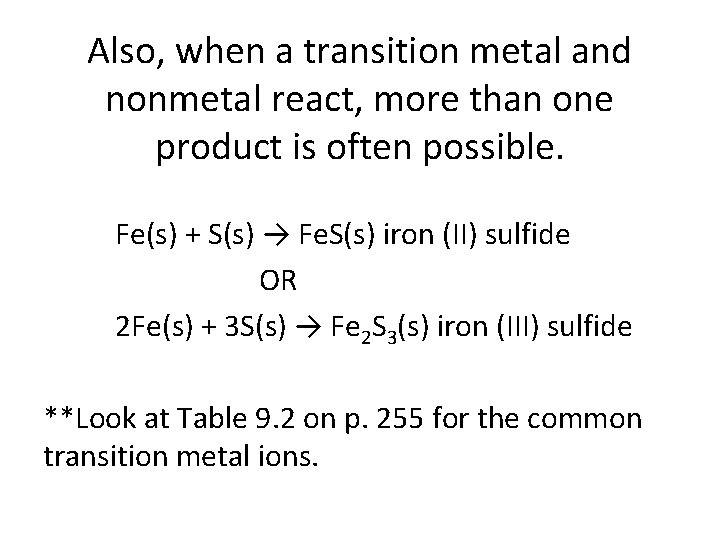
Also, when a transition metal and nonmetal react, more than one product is often possible. Fe(s) + S(s) → Fe. S(s) iron (II) sulfide OR 2 Fe(s) + 3 S(s) → Fe 2 S 3(s) iron (III) sulfide **Look at Table 9. 2 on p. 255 for the common transition metal ions.
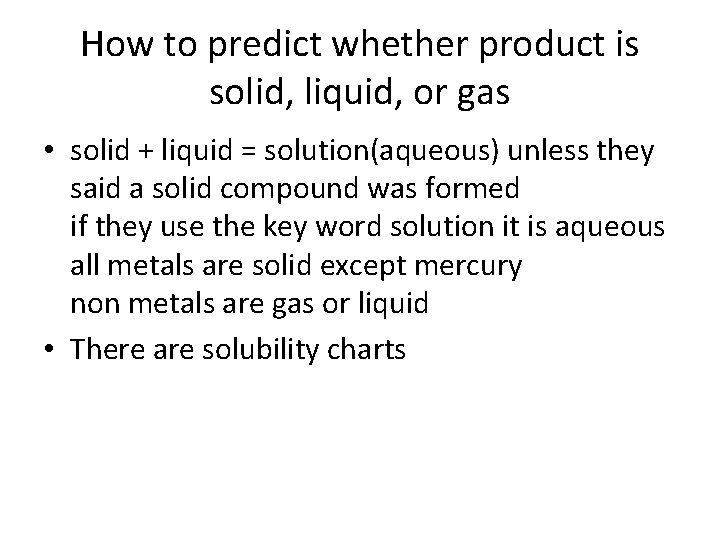
How to predict whether product is solid, liquid, or gas • solid + liquid = solution(aqueous) unless they said a solid compound was formed if they use the key word solution it is aqueous all metals are solid except mercury non metals are gas or liquid • There are solubility charts
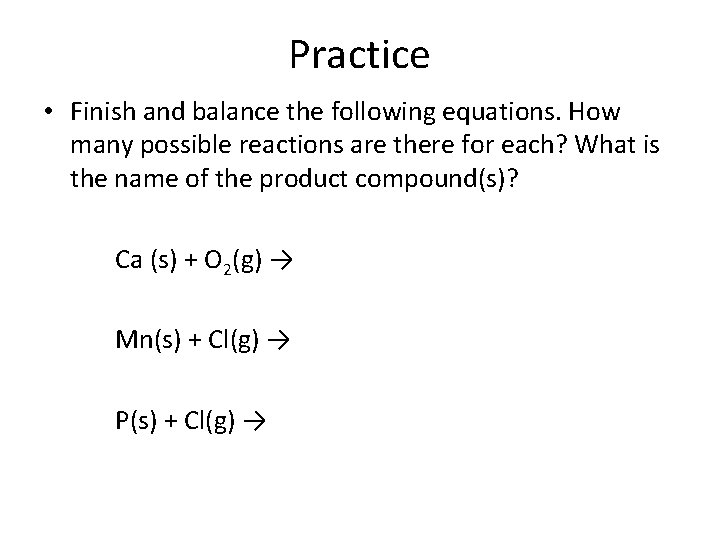
Practice • Finish and balance the following equations. How many possible reactions are there for each? What is the name of the product compound(s)? Ca (s) + O 2(g) → Mn(s) + Cl(g) → P(s) + Cl(g) →
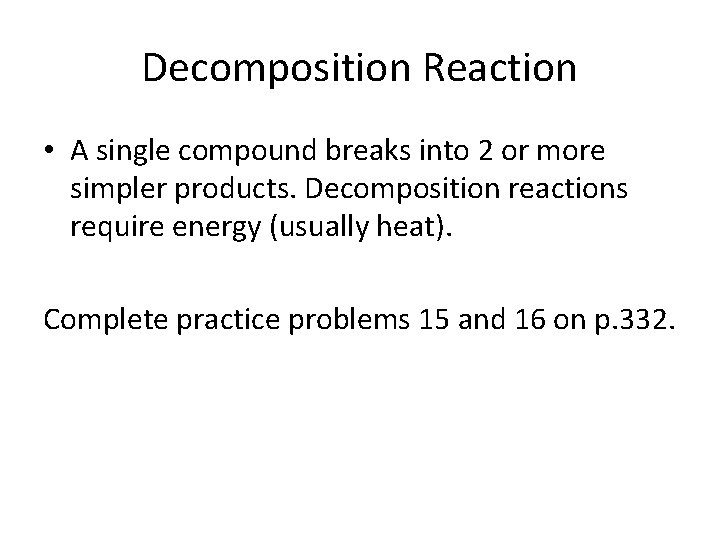
Decomposition Reaction • A single compound breaks into 2 or more simpler products. Decomposition reactions require energy (usually heat). Complete practice problems 15 and 16 on p. 332.

• Complete and balance the decomposition reaction HI →
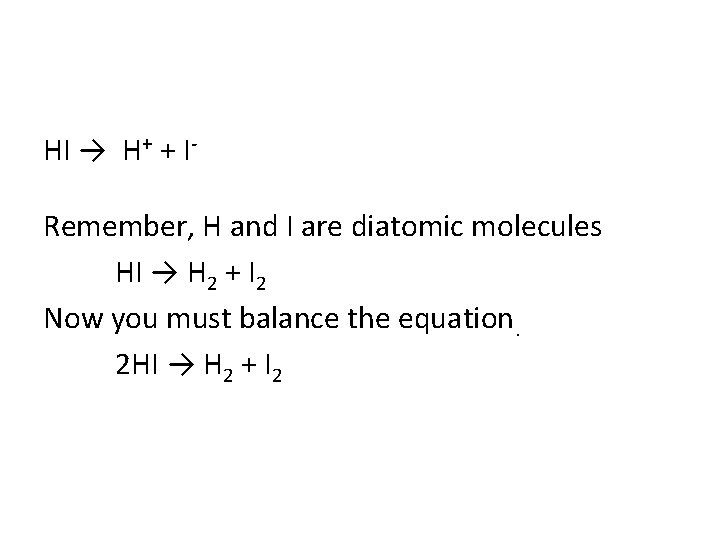
HI → H+ + IRemember, H and I are diatomic molecules HI → H 2 + I 2 Now you must balance the equation. 2 HI → H 2 + I 2

Write the formula for the binary compound that decomposes to the products H 2 and Br 2.

Since H is 1+ and Br is 1+, the compound that forms H 2 and Br 2 is HBr
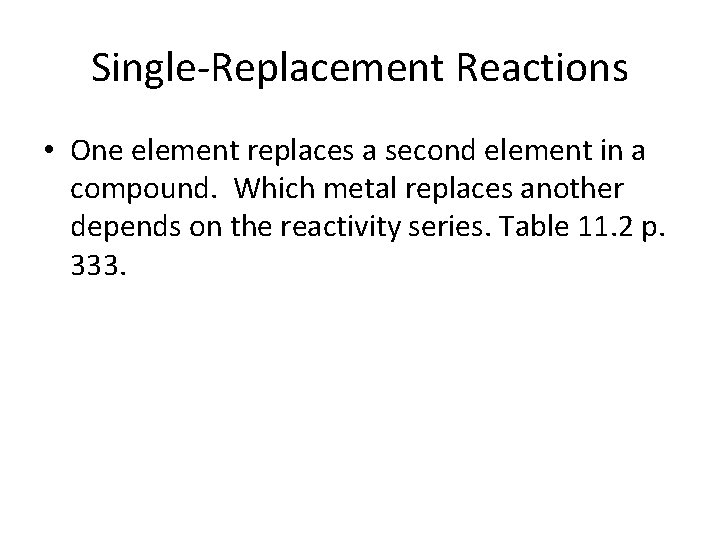
Single-Replacement Reactions • One element replaces a second element in a compound. Which metal replaces another depends on the reactivity series. Table 11. 2 p. 333.
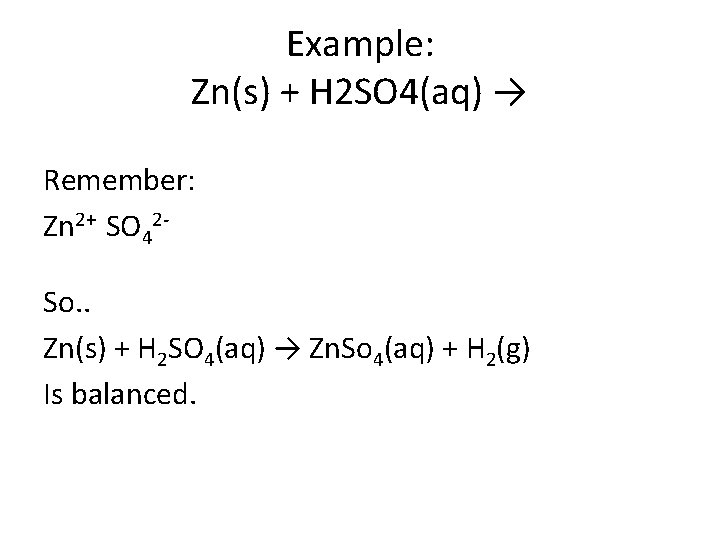
Example: Zn(s) + H 2 SO 4(aq) → Remember: Zn 2+ SO 42 So. . Zn(s) + H 2 SO 4(aq) → Zn. So 4(aq) + H 2(g) Is balanced.
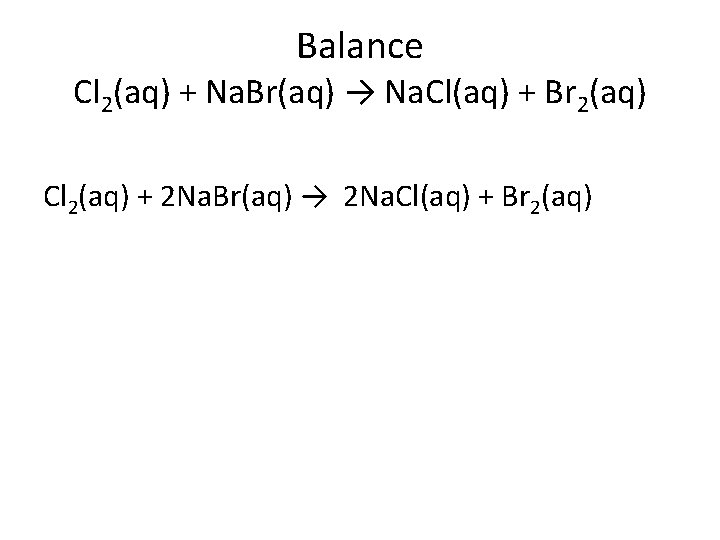
Balance Cl 2(aq) + Na. Br(aq) → Na. Cl(aq) + Br 2(aq) Cl 2(aq) + 2 Na. Br(aq) → 2 Na. Cl(aq) + Br 2(aq)

Do Practice Problem 17 on p. 334 in groups. Answers are on next slides
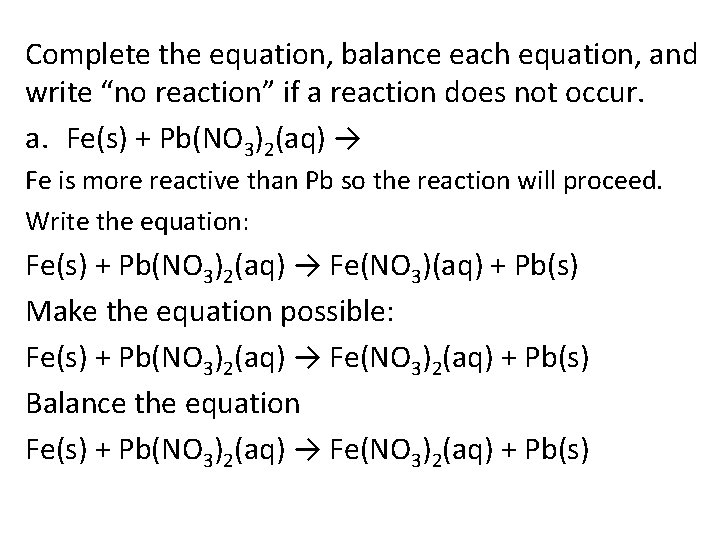
Complete the equation, balance each equation, and write “no reaction” if a reaction does not occur. a. Fe(s) + Pb(NO 3)2(aq) → Fe is more reactive than Pb so the reaction will proceed. Write the equation: Fe(s) + Pb(NO 3)2(aq) → Fe(NO 3)(aq) + Pb(s) Make the equation possible: Fe(s) + Pb(NO 3)2(aq) → Fe(NO 3)2(aq) + Pb(s) Balance the equation Fe(s) + Pb(NO 3)2(aq) → Fe(NO 3)2(aq) + Pb(s)
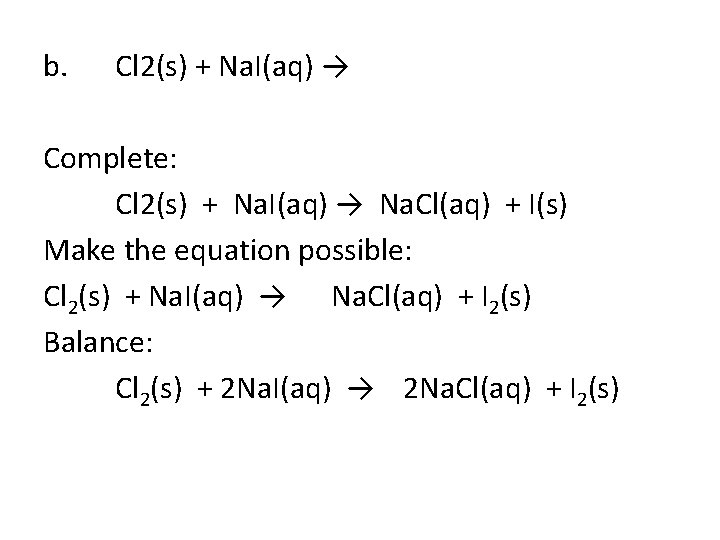
b. Cl 2(s) + Na. I(aq) → Complete: Cl 2(s) + Na. I(aq) → Na. Cl(aq) + I(s) Make the equation possible: Cl 2(s) + Na. I(aq) → Na. Cl(aq) + I 2(s) Balance: Cl 2(s) + 2 Na. I(aq) → 2 Na. Cl(aq) + I 2(s)
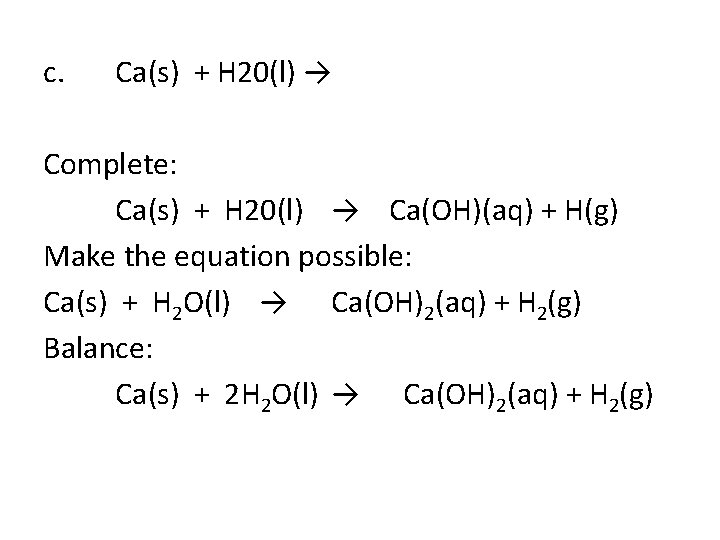
c. Ca(s) + H 20(l) → Complete: Ca(s) + H 20(l) → Ca(OH)(aq) + H(g) Make the equation possible: Ca(s) + H 2 O(l) → Ca(OH)2(aq) + H 2(g) Balance: Ca(s) + 2 H 2 O(l) → Ca(OH)2(aq) + H 2(g)
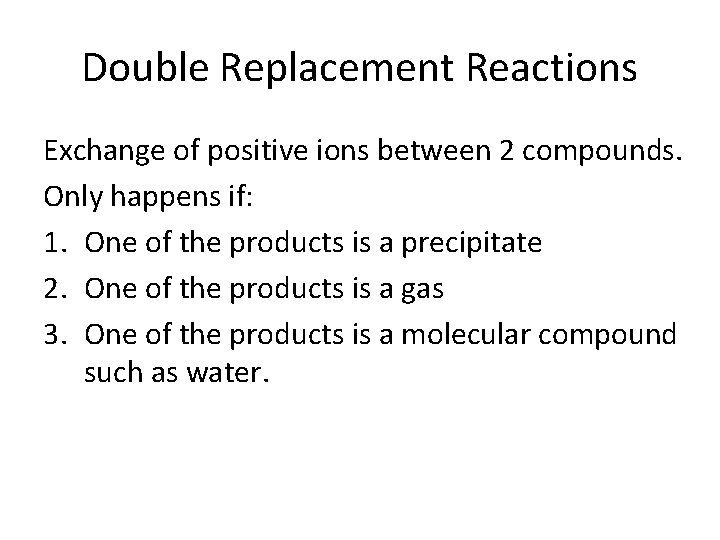
Double Replacement Reactions Exchange of positive ions between 2 compounds. Only happens if: 1. One of the products is a precipitate 2. One of the products is a gas 3. One of the products is a molecular compound such as water.
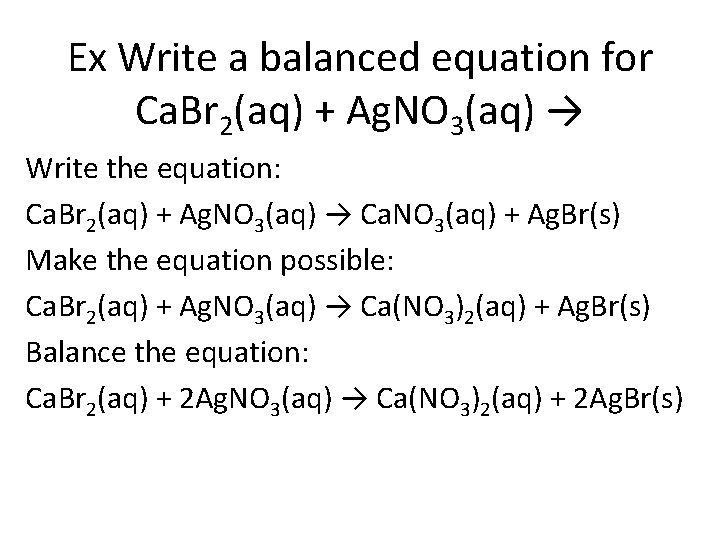
Ex Write a balanced equation for Ca. Br 2(aq) + Ag. NO 3(aq) → Write the equation: Ca. Br 2(aq) + Ag. NO 3(aq) → Ca. NO 3(aq) + Ag. Br(s) Make the equation possible: Ca. Br 2(aq) + Ag. NO 3(aq) → Ca(NO 3)2(aq) + Ag. Br(s) Balance the equation: Ca. Br 2(aq) + 2 Ag. NO 3(aq) → Ca(NO 3)2(aq) + 2 Ag. Br(s)
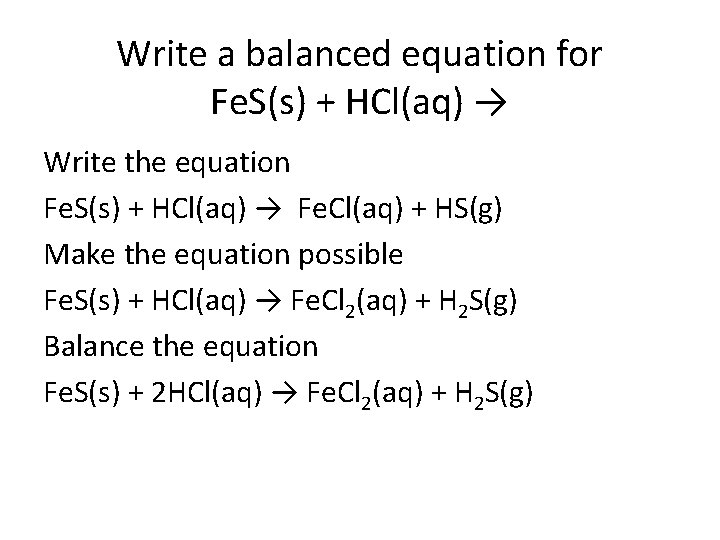
Write a balanced equation for Fe. S(s) + HCl(aq) → Write the equation Fe. S(s) + HCl(aq) → Fe. Cl(aq) + HS(g) Make the equation possible Fe. S(s) + HCl(aq) → Fe. Cl 2(aq) + H 2 S(g) Balance the equation Fe. S(s) + 2 HCl(aq) → Fe. Cl 2(aq) + H 2 S(g)

Practice Problems p. 335 • Answers on next slides
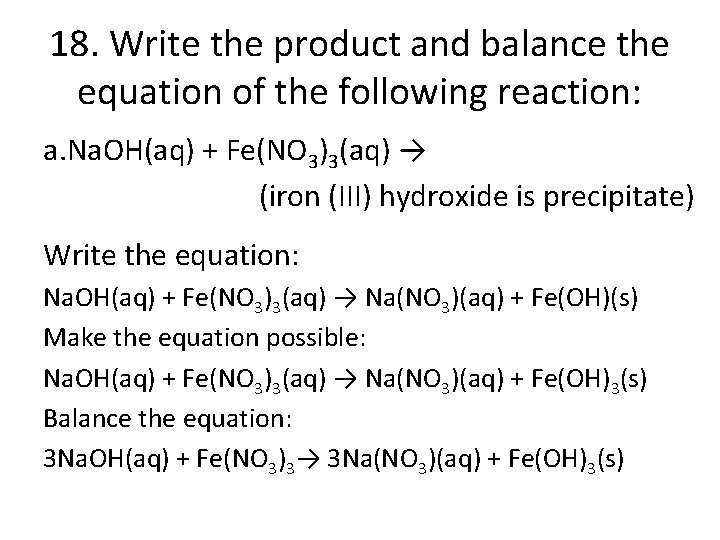
18. Write the product and balance the equation of the following reaction: a. Na. OH(aq) + Fe(NO 3)3(aq) → (iron (III) hydroxide is precipitate) Write the equation: Na. OH(aq) + Fe(NO 3)3(aq) → Na(NO 3)(aq) + Fe(OH)(s) Make the equation possible: Na. OH(aq) + Fe(NO 3)3(aq) → Na(NO 3)(aq) + Fe(OH)3(s) Balance the equation: 3 Na. OH(aq) + Fe(NO 3)3→ 3 Na(NO 3)(aq) + Fe(OH)3(s)
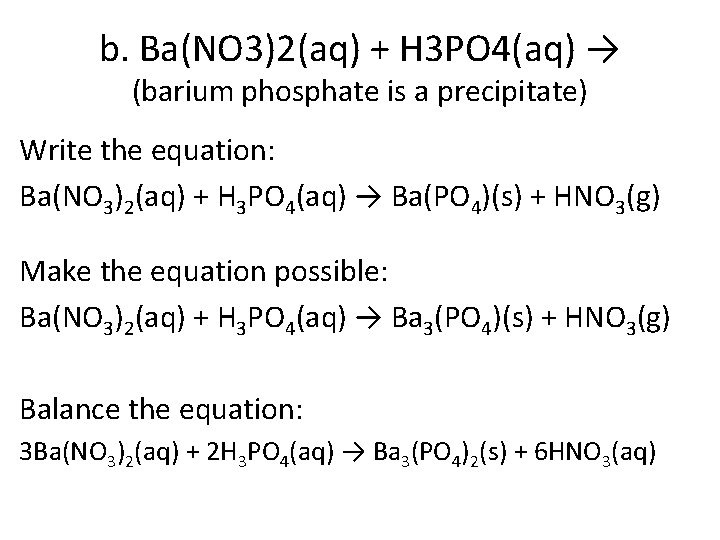
b. Ba(NO 3)2(aq) + H 3 PO 4(aq) → (barium phosphate is a precipitate) Write the equation: Ba(NO 3)2(aq) + H 3 PO 4(aq) → Ba(PO 4)(s) + HNO 3(g) Make the equation possible: Ba(NO 3)2(aq) + H 3 PO 4(aq) → Ba 3(PO 4)(s) + HNO 3(g) Balance the equation: 3 Ba(NO 3)2(aq) + 2 H 3 PO 4(aq) → Ba 3(PO 4)2(s) + 6 HNO 3(aq)
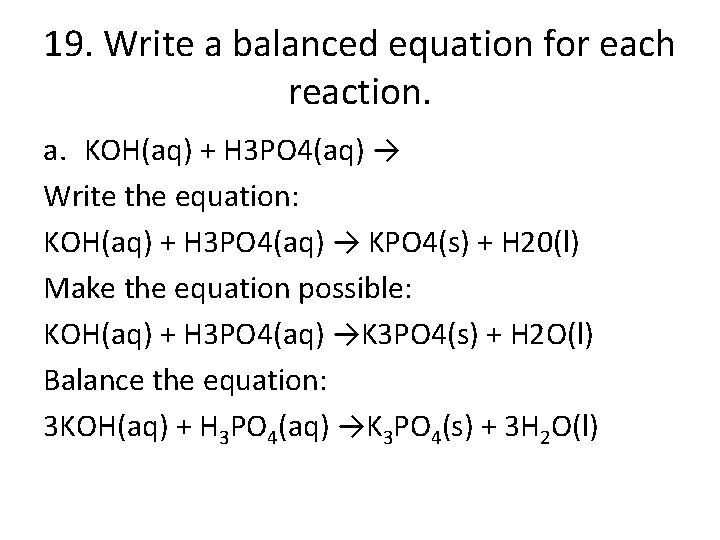
19. Write a balanced equation for each reaction. a. KOH(aq) + H 3 PO 4(aq) → Write the equation: KOH(aq) + H 3 PO 4(aq) → KPO 4(s) + H 20(l) Make the equation possible: KOH(aq) + H 3 PO 4(aq) →K 3 PO 4(s) + H 2 O(l) Balance the equation: 3 KOH(aq) + H 3 PO 4(aq) →K 3 PO 4(s) + 3 H 2 O(l)

19 b. H 2 SO 4(aq) + Al(OH)3(aq) → Write the equation: H 2 SO 4(aq) + Al(OH)3(aq) → Al. SO 4(g) + H 2 O(l) Make the equation possible: H 2 SO 4(aq) + Al(OH)3(aq) → Al 2(SO 4)3(g) + H 2 O(l) Balance the equation: 3 H 2 SO 4(aq) + 2 Al(OH)3(aq) → Al 2(SO 4)3(g) + 6 H 2 O(l)
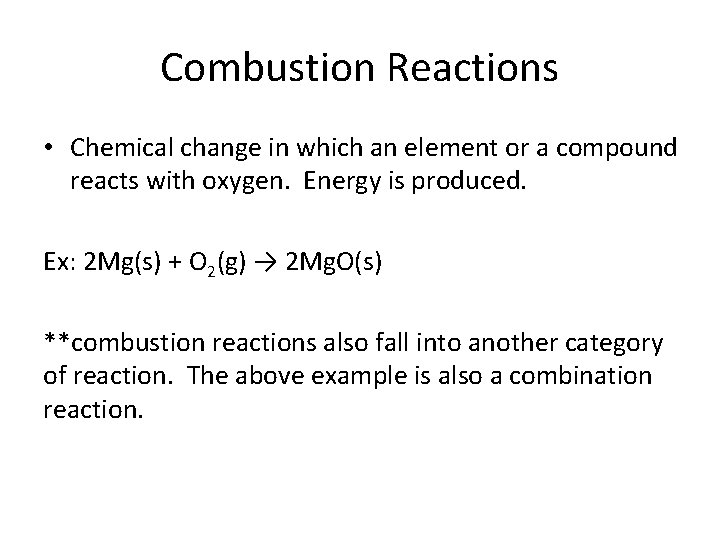
Combustion Reactions • Chemical change in which an element or a compound reacts with oxygen. Energy is produced. Ex: 2 Mg(s) + O 2(g) → 2 Mg. O(s) **combustion reactions also fall into another category of reaction. The above example is also a combination reaction.
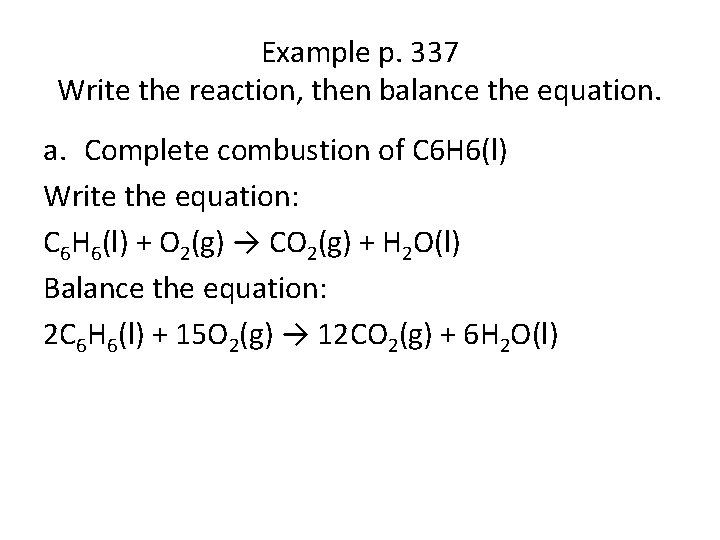
Example p. 337 Write the reaction, then balance the equation. a. Complete combustion of C 6 H 6(l) Write the equation: C 6 H 6(l) + O 2(g) → CO 2(g) + H 2 O(l) Balance the equation: 2 C 6 H 6(l) + 15 O 2(g) → 12 CO 2(g) + 6 H 2 O(l)
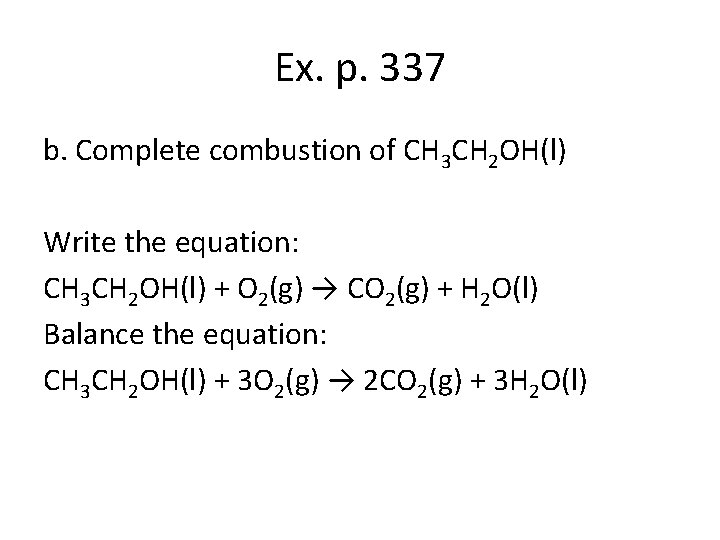
Ex. p. 337 b. Complete combustion of CH 3 CH 2 OH(l) Write the equation: CH 3 CH 2 OH(l) + O 2(g) → CO 2(g) + H 2 O(l) Balance the equation: CH 3 CH 2 OH(l) + 3 O 2(g) → 2 CO 2(g) + 3 H 2 O(l)

Do Practice Problems p. 337 20, 21 • Answers on next slide

337 #20 Write a balance equation for the combustion of a. HCOOH Write the equation: HCOOH + O 2(g) → CO 2(g) + H 2 O(l) Balance the equation: 2 HCOOH + 2 O 2(g) → 2 CO 2(g) + 2 H 2 O(l)

b. Write a balanced equation for the combustion of C 7 H 16. Write the equation: C 7 H 16(l) + O 2(g) → CO 2(g) + H 2 O(l) Balance the equation: C 7 H 16(l) + 11 O 2(g) → 7 CO 2(g) + 8 H 2 O(l)
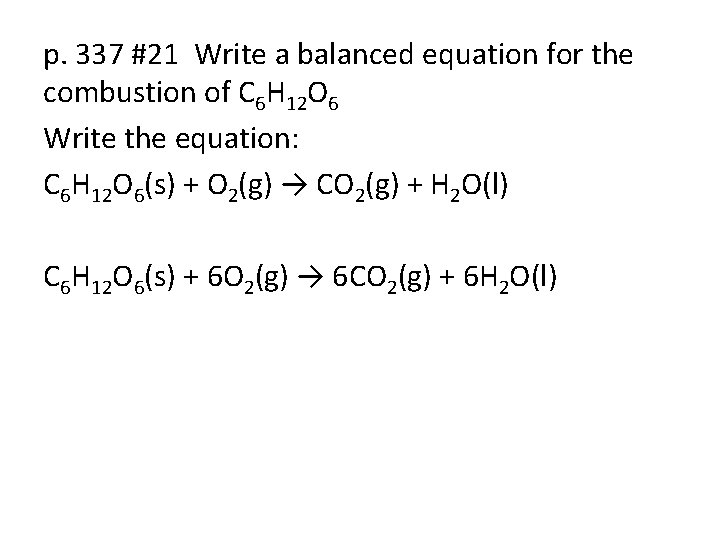
p. 337 #21 Write a balanced equation for the combustion of C 6 H 12 O 6 Write the equation: C 6 H 12 O 6(s) + O 2(g) → CO 2(g) + H 2 O(l) C 6 H 12 O 6(s) + 6 O 2(g) → 6 CO 2(g) + 6 H 2 O(l)

• Homework: • P. 339 # 22 -27 • Worksheet
- Slides: 34