CHEMISTRY NOTES ELEMENTS THE PERIODIC TABLE Originally organized
CHEMISTRY NOTES : ELEMENTS

THE PERIODIC TABLE • Originally organized by increasing atomic mass by Isaac Mendeleev in 1869. – Later organized left to right by increasing atomic # (by Moseley in early 1900’s). • Each column (up and down) contains elements with similar chemical properties. • The elements are categorized into three groups: metals, nonmetals and metalloids. • The elements that have an atomic number above 93 are made in a lab; the first 92 can be found in nature.

ELEMENTS • Elements CANNOT be broken down into simpler substances, and are made of only one kind of atom. • Elements are the building blocks of matter. • Some elements in everyday life: –Oxygen – to breathe –Silicon – used in computer chips –Gold and silver – jewelry –Carbon – found in all living things
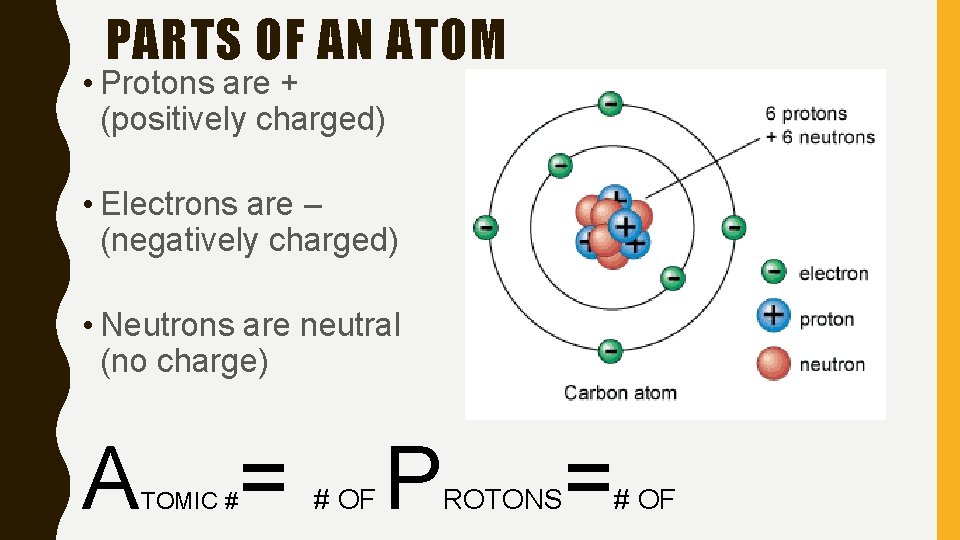
PARTS OF AN ATOM • Protons are + (positively charged) • Electrons are – (negatively charged) • Neutrons are neutral (no charge) A TOMIC # = # OF P ROTONS = # OF
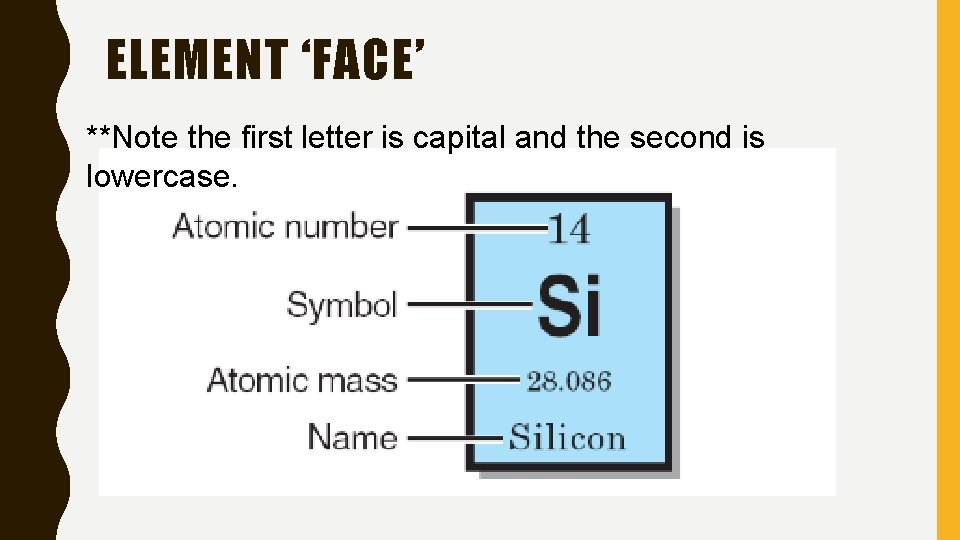
ELEMENT ‘FACE’ **Note the first letter is capital and the second is lowercase.
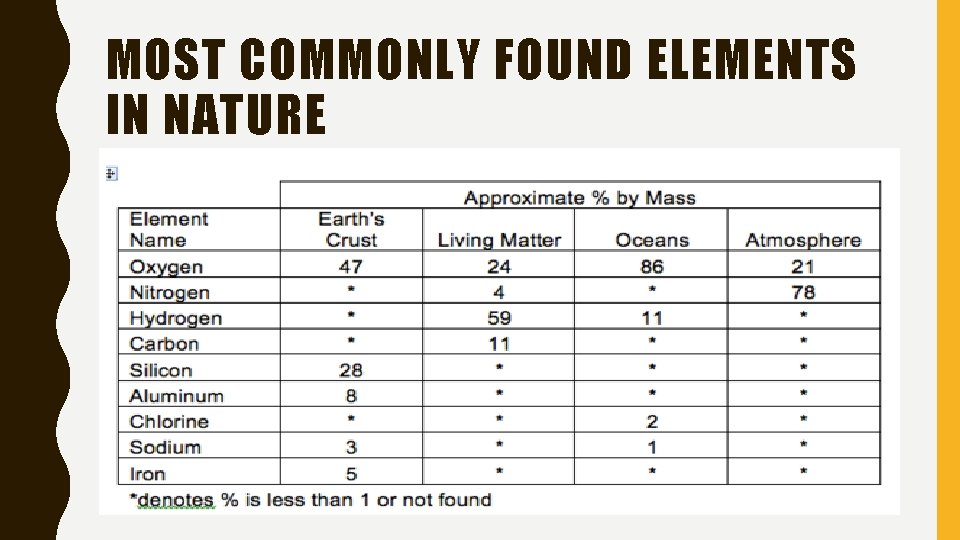
MOST COMMONLY FOUND ELEMENTS IN NATURE

PROPERTIES OF METALS • Most are solid at room temperature • metallic luster(shiny) • Metals are Ductile (can be drawn into thin wire) – Copper wiring • Malleable (can be hammered into sheets) like Tin foil • Metals have a high melting point. • very dense. • Metals are good conductors of electricity and heat • A chemical property of metal is its reaction with water and oxygen. This results in corrosion, tarnish and rust
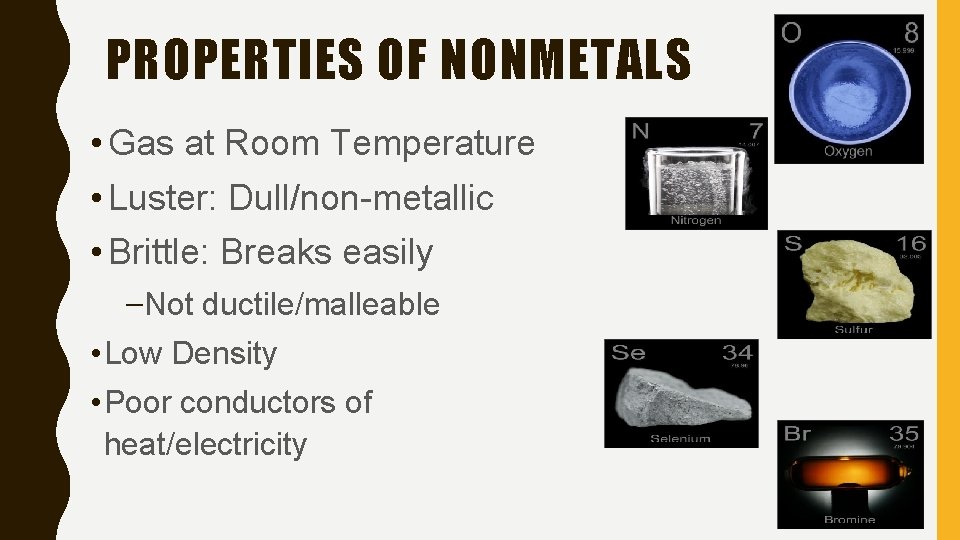
PROPERTIES OF NONMETALS • Gas at Room Temperature • Luster: Dull/non-metallic • Brittle: Breaks easily –Not ductile/malleable • Low Density • Poor conductors of heat/electricity

PROPERTIES OF METALLOIDS • **Have properties of both metals and nonmetals** • Solids can be either shiny or dull • Malleable and ductile (but not as much as metals) • Conduct electricity and heat better than nonmetals but not as well as metals
- Slides: 9