Chemistry Molecules of Life Unit 2 AtomsBuilding Blocks

Chemistry: Molecules of Life Unit 2
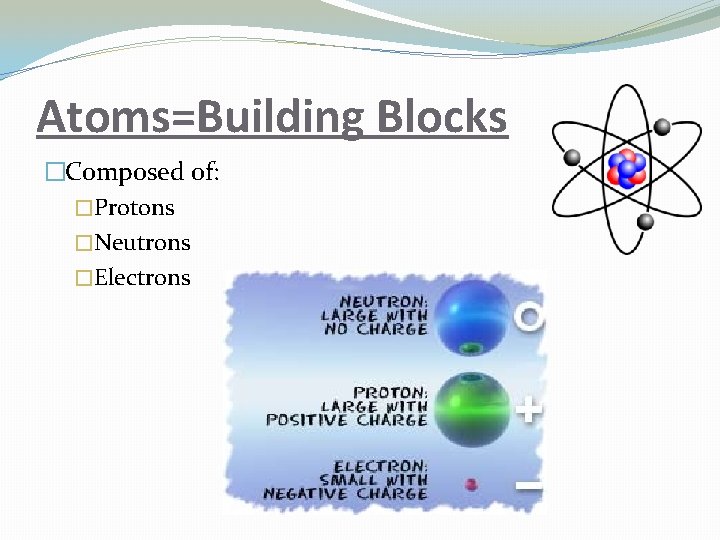
Atoms=Building Blocks �Composed of: �Protons �Neutrons �Electrons
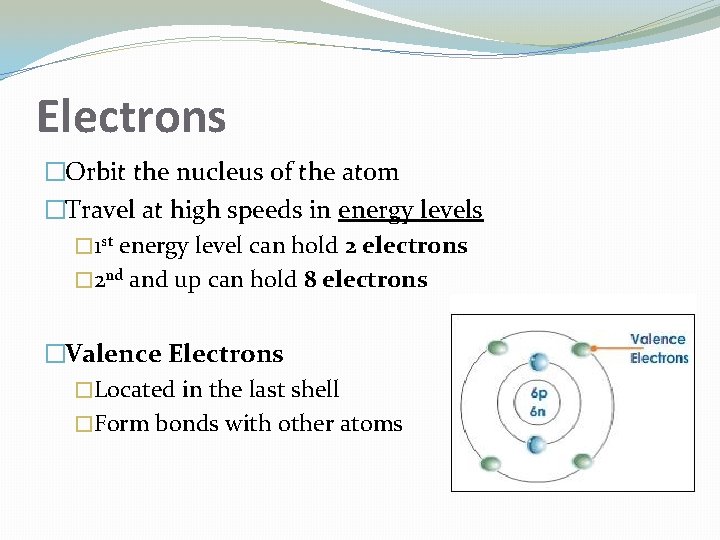
Electrons �Orbit the nucleus of the atom �Travel at high speeds in energy levels � 1 st energy level can hold 2 electrons � 2 nd and up can hold 8 electrons �Valence Electrons �Located in the last shell �Form bonds with other atoms
Elements �Composed of only one: �ATOM �They are pure substances that can‘t be broken down
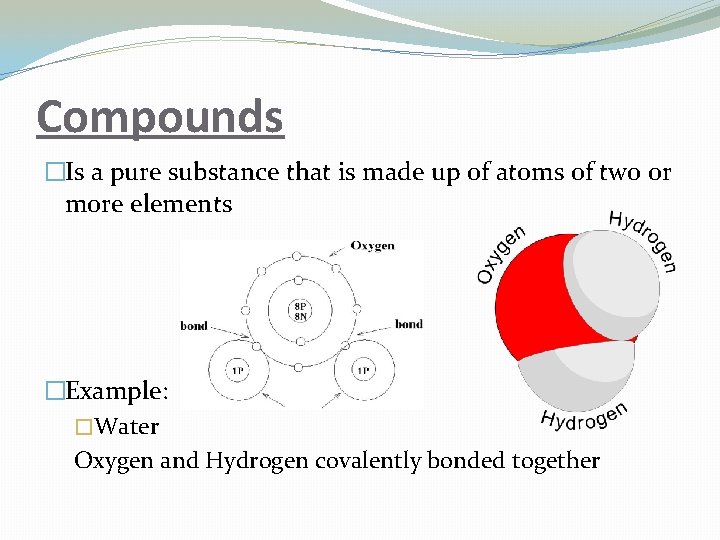
Compounds �Is a pure substance that is made up of atoms of two or more elements �Example: �Water Oxygen and Hydrogen covalently bonded together
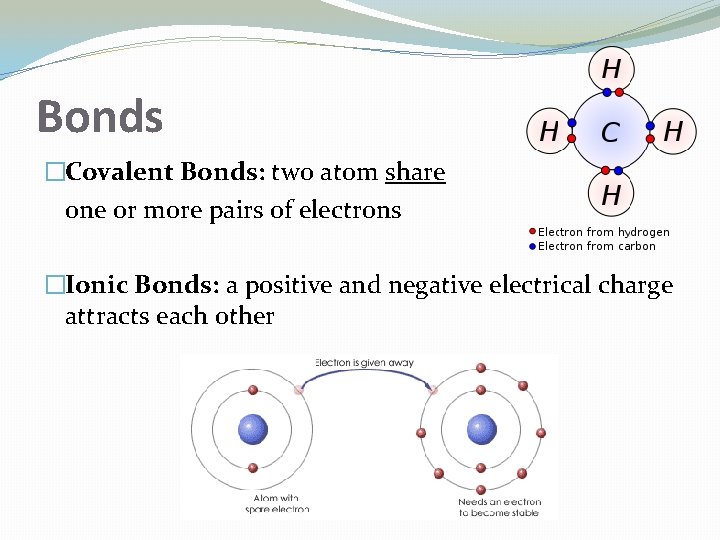
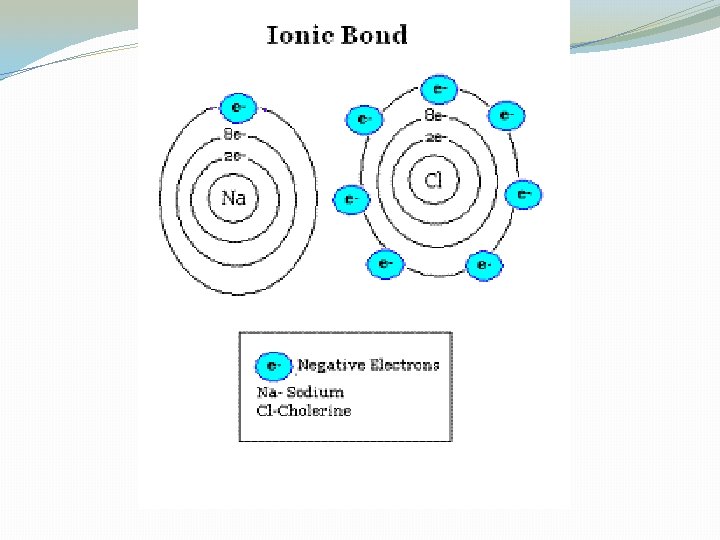
Bonds �Covalent Bonds: two atom share one or more pairs of electrons �Ionic Bonds: a positive and negative electrical charge attracts each other

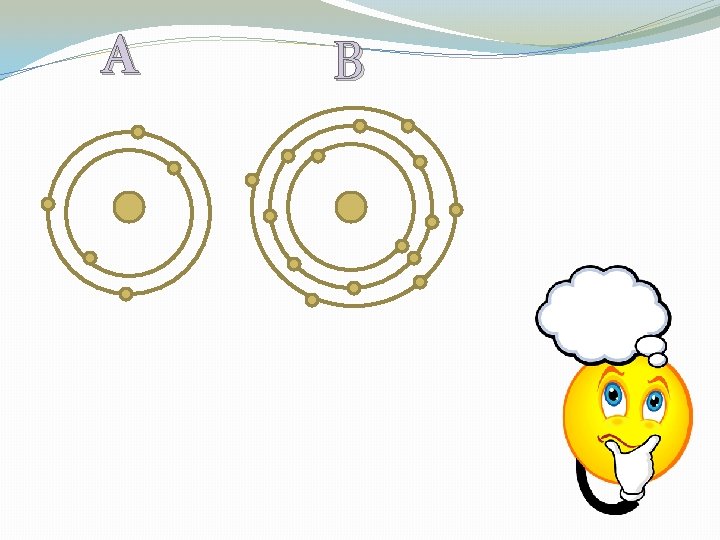
�Having 8 valence electrons = stable

Which Atom is stable?
A B

Polarity �Non-Polar: A compound comprised of molecules linked through chemical bonds arranged in such a way that the distribution of charges are symmetrical Example: Fats, Oil and Gasoline Water-insoluble (hydrophobic): water “hating”
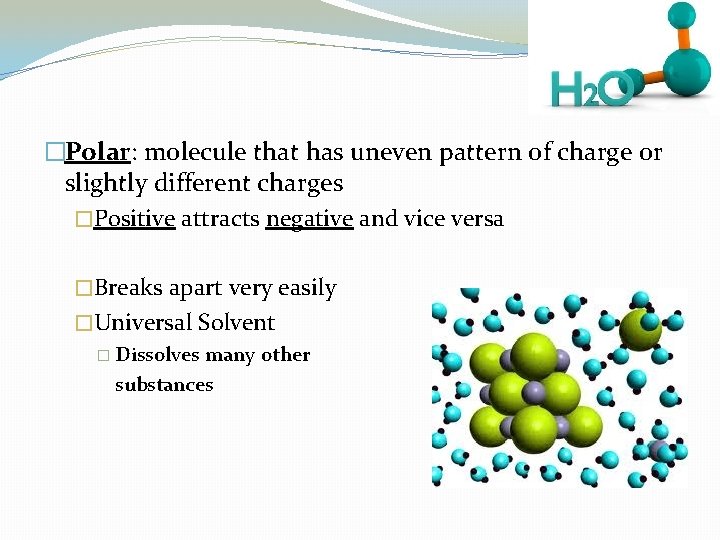
�Polar: molecule that has uneven pattern of charge or slightly different charges �Positive attracts negative and vice versa �Breaks apart very easily �Universal Solvent � Dissolves many other substances
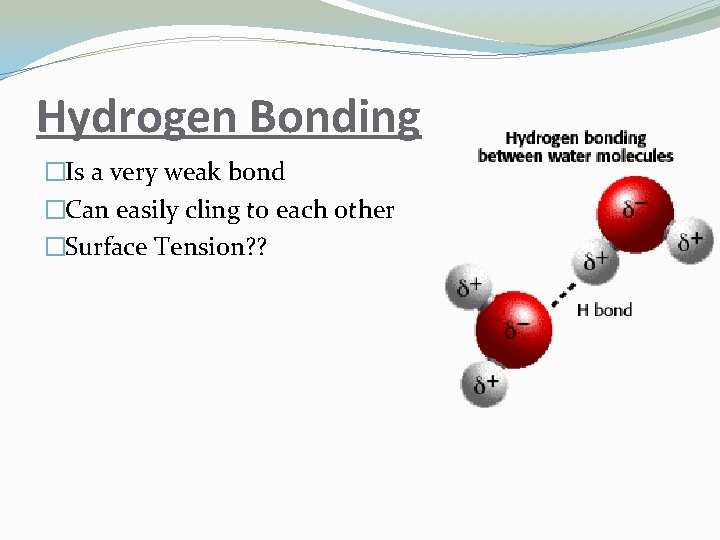
Hydrogen Bonding �Is a very weak bond �Can easily cling to each other �Surface Tension? ?

Cohesion �An attractive force between particle of the same kind. �Surface Tension �Filling water to the brim of a glass

Adhesion �An attractive force between unlike substances. �Balloon and water demo �Meniscus in the graduated cylinder �Water sticky to wooden desk

Capillarity �Adhesion and cohesion enable water to move upwards against gravity �Water from soil (or cup) to leaves and stem

Carbohydrates

Carbs �Organic compounds: �Carbon �Hydrogen �Oxygen �Exist with a ration of 2 hydrogen (H) atoms to 1 oxygen (O) atom �Essential to the life process of all living things, provide energy

Did you know. . . �Carbohydrates are: �Bread �Sugar � Milk � Fruits � Soda �Pasta �Cereal �Beans �Potatoes �Glycogen �Starch

There are 3 Flavors �Monosaccharide �Disaccharide �Polysaccharide
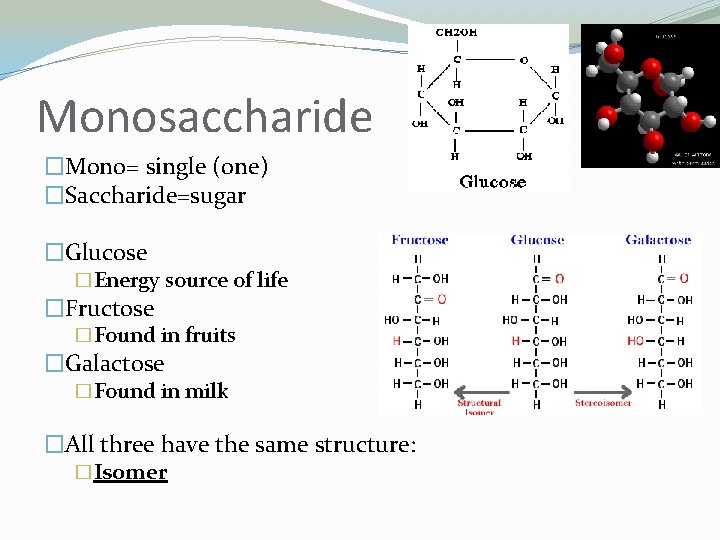
Monosaccharide �Mono= single (one) �Saccharide=sugar �Glucose �Energy source of life �Fructose �Found in fruits �Galactose �Found in milk �All three have the same structure: �Isomer
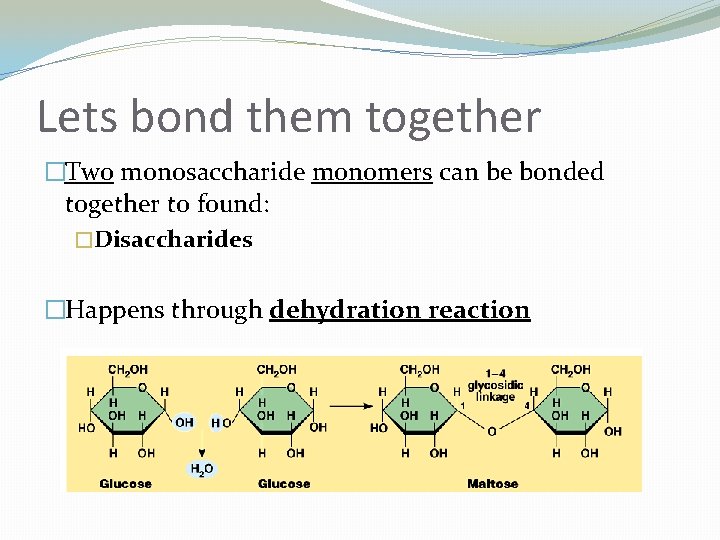
Lets bond them together �Two monosaccharide monomers can be bonded together to found: �Disaccharides �Happens through dehydration reaction
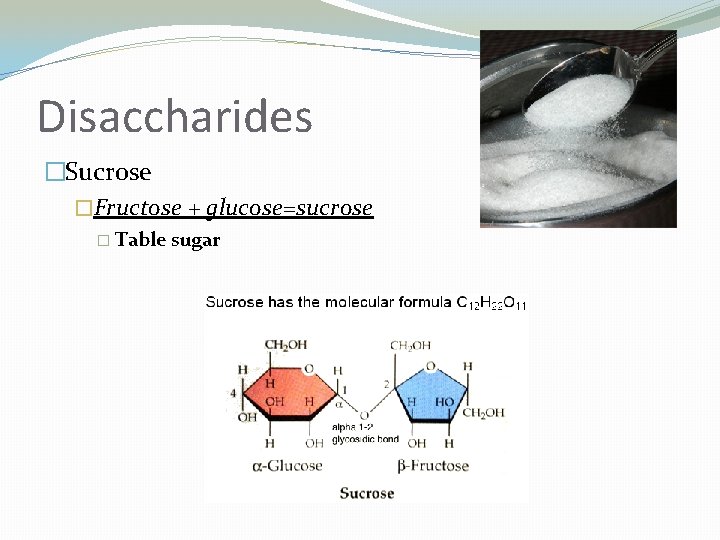
Disaccharides �Sucrose �Fructose + glucose=sucrose � Table sugar
Polysaccharide �Complex molecule composed of 3 or more monosaccharides �Usually 100 -1000 (very long structures) �Spaghetti is 1000 s of glucose molecules strung together!! � Used for storage in both plants and animals �Starch: plants �Glycogen: humans �Used for support: �Cellulose in plant cells �Chitin in scorpion’s exoskeleton
Proteins
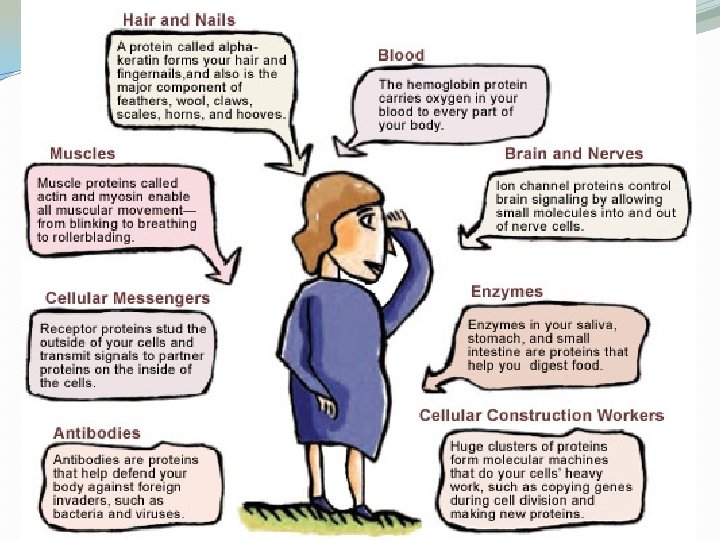
Proteins �Organic compounds: �Carbon �Hydrogen �Nitrogen �Oxygen �Essential for many parts of the body: �Skin and muscles �Walking and eating �Tanning �Making hemoglobin �Many, many more things

What does You. Tube say? �https: //www. youtube. com/watch? v=wctk. PUUp. Uc&feature=player_embedded
Proteins �“polypeptides” �Polymers of amino acids joined by peptide bonds �Joining of amino acids to create: �Dipeptide �Polypeptide �Done through a dehydration reaction (so what is being lost? ) �Proteins are composed of one or more polypeptides
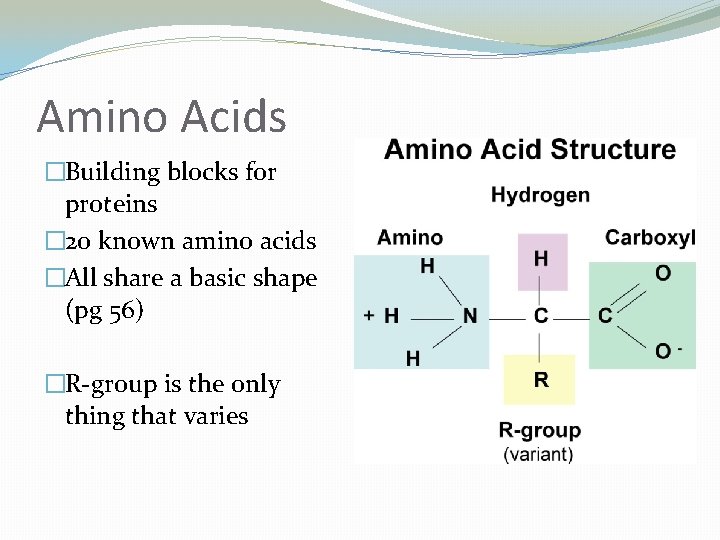
Amino Acids �Building blocks for proteins � 20 known amino acids �All share a basic shape (pg 56) �R-group is the only thing that varies
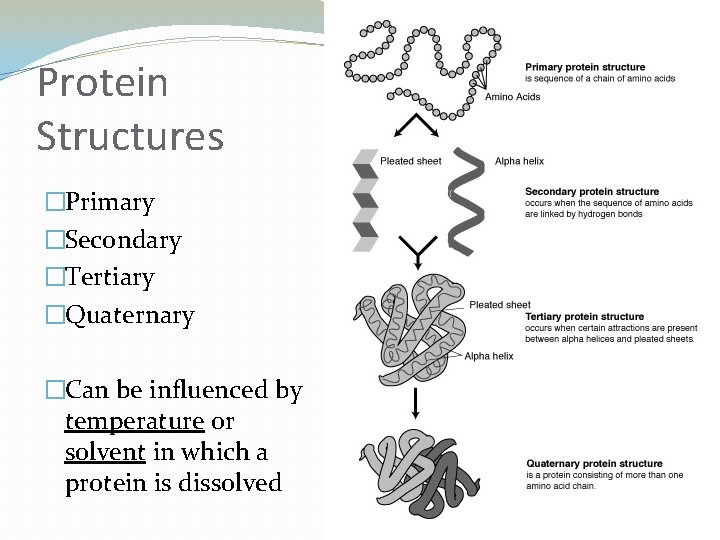
Protein Structures �Primary �Secondary �Tertiary �Quaternary �Can be influenced by temperature or solvent in which a protein is dissolved
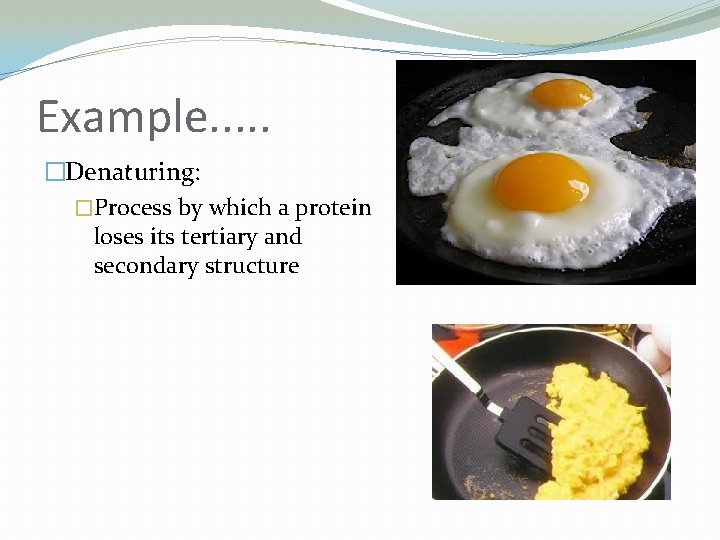
Example. . . �Denaturing: �Process by which a protein loses its tertiary and secondary structure

Nucleic Acids
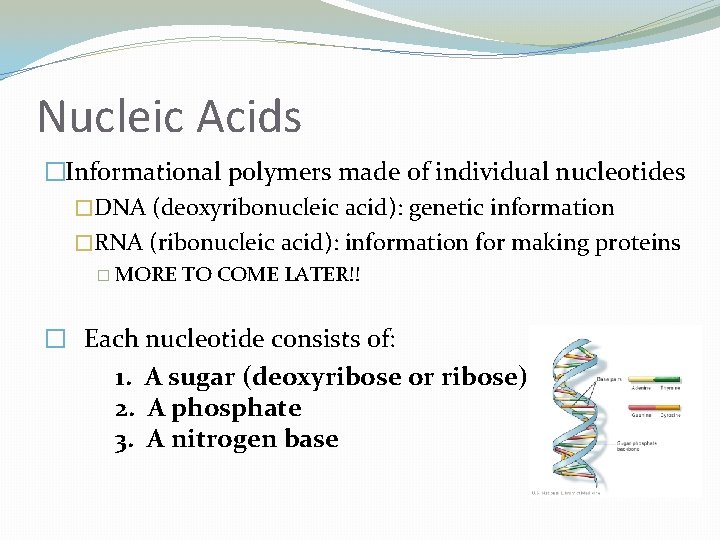
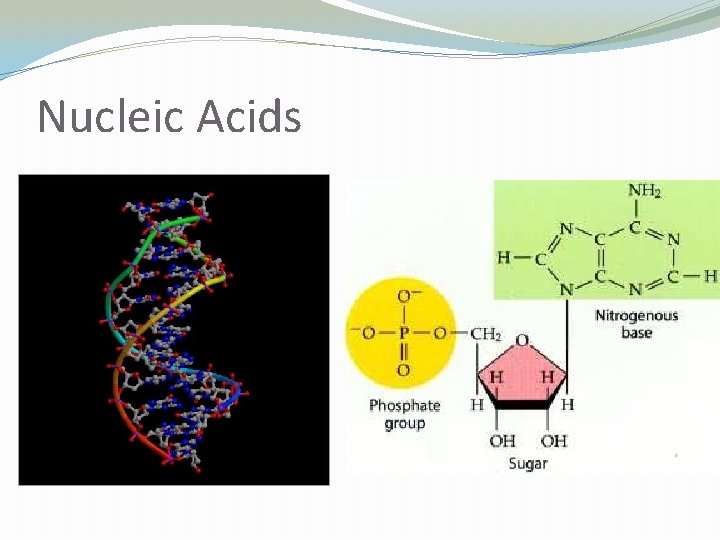
Nucleic Acids �Informational polymers made of individual nucleotides �DNA (deoxyribonucleic acid): genetic information �RNA (ribonucleic acid): information for making proteins � MORE TO COME LATER!! � Each nucleotide consists of: 1. A sugar (deoxyribose or ribose) 2. A phosphate 3. A nitrogen base
Nucleic Acids

Lipids

Lipids �Nonpolar organic molecules that do not dissolve in water. �Carbon �Oxygen �Hydrogen �Effective at storing energy; better than sugar �But dietary fats can be dangerous to your health
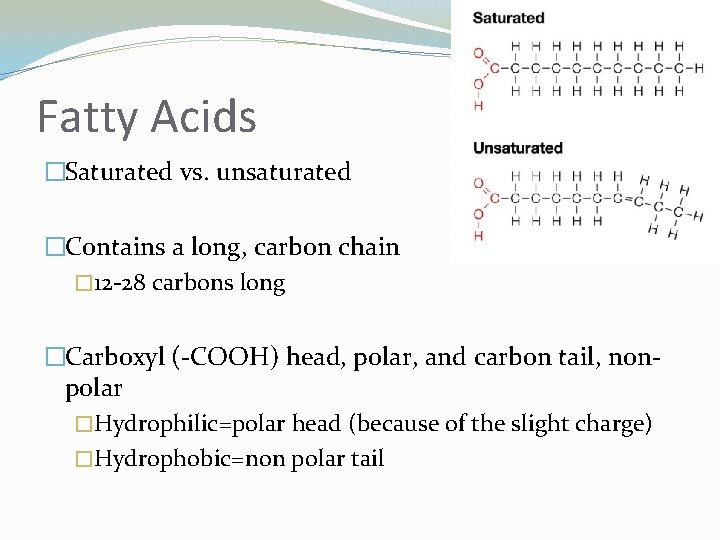
Fatty Acids �Saturated vs. unsaturated �Contains a long, carbon chain � 12 -28 carbons long �Carboxyl (-COOH) head, polar, and carbon tail, nonpolar �Hydrophilic=polar head (because of the slight charge) �Hydrophobic=non polar tail
Unsaturated Fatty Acids (The Good Fat) �“unsaturated” with hydrogen bonds �Because of the double bond �Have kinks where there is a double bond �Causes the molecule to be more fluid (doesn’t clog arteries) �Liquid at room temperature

Even healthier �Monounsaturated fatty acids �One double bond �Olive oil �Polyunsaturated fatty acids �Many double bonds �Omega-3 fatty acids (fish oil)
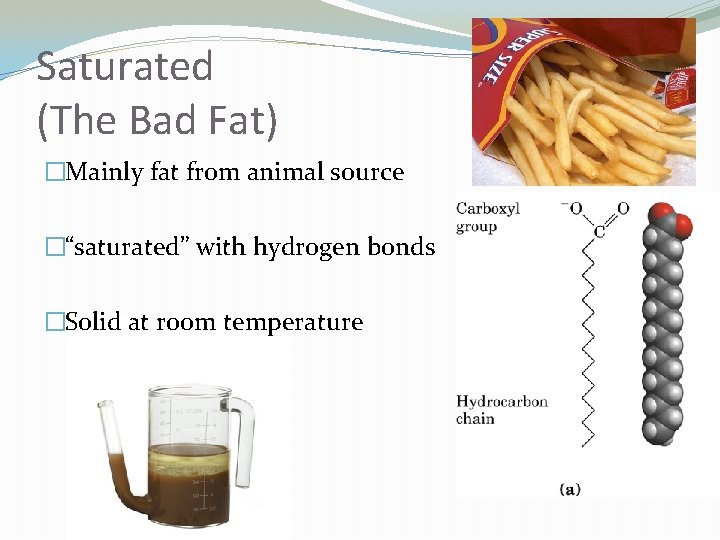
Saturated (The Bad Fat) �Mainly fat from animal source �“saturated” with hydrogen bonds �Solid at room temperature

Trans Fat �Also known as a “bad” fat �Occurs naturally in some animal fat but most are made in the lab

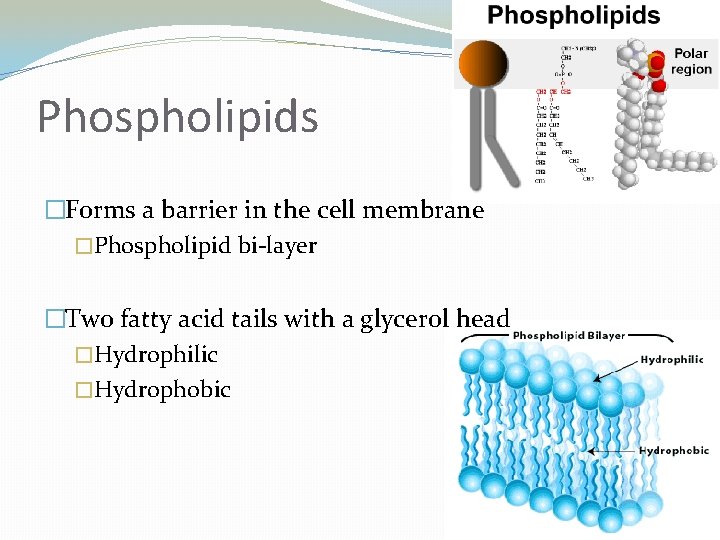
Phospholipids �Forms a barrier in the cell membrane �Phospholipid bi-layer �Two fatty acid tails with a glycerol head �Hydrophilic �Hydrophobic
Other types of lipids �Wax �Forms protection for ears, bees, leaves of plants. . . and more �Steroids �Composed of four carbon rings �Examples � Hormones � Cholesterol

Nutritional Facts
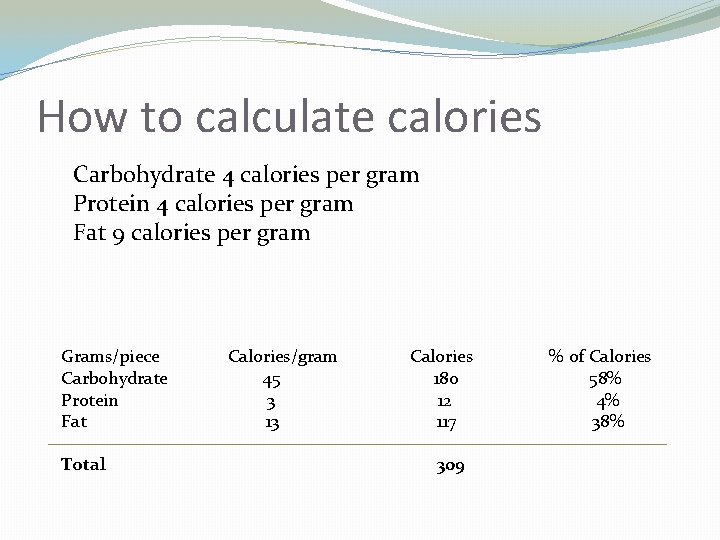
How to calculate calories Carbohydrate 4 calories per gram Protein 4 calories per gram Fat 9 calories per gram Grams/piece Carbohydrate Protein Fat Total Calories/gram 45 3 13 Calories 180 12 117 309 % of Calories 58% 4% 38%

�Recommended daily amounts: Carbohydrate= 45 -65% Protein= 10 -35% Fat= 20 -35
- Slides: 48