Chemistry Math Mini Unit Measurement a quantity that










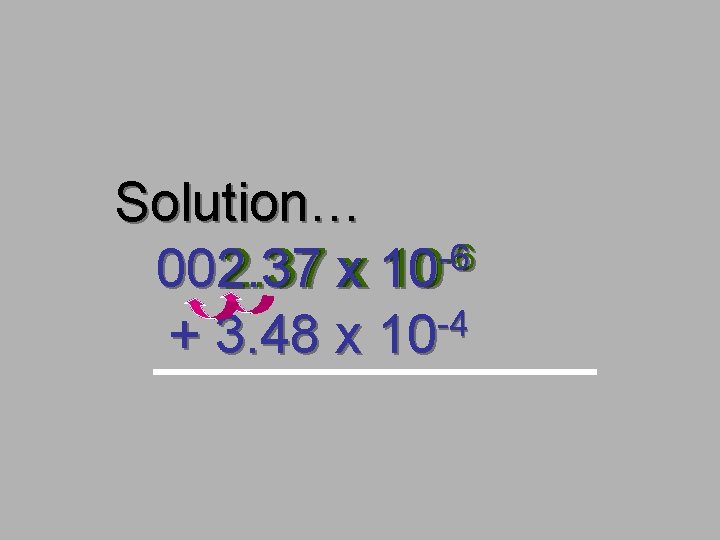
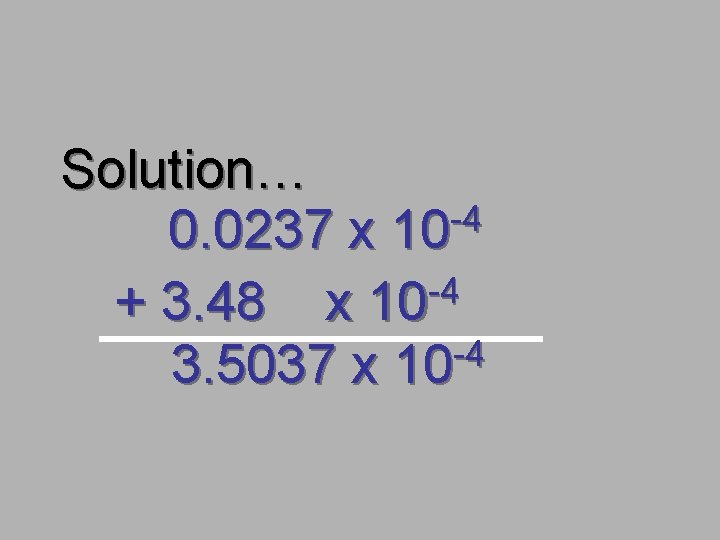


































- Slides: 77

Chemistry Math Mini Unit

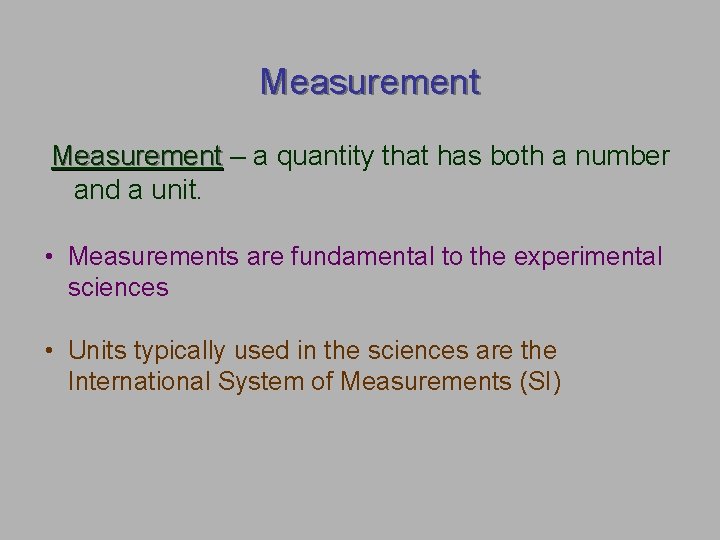
Measurement – a quantity that has both a number and a unit. • Measurements are fundamental to the experimental sciences • Units typically used in the sciences are the International System of Measurements (SI)

Scientific Notation In science, we deal with some very LARGE numbers: 1 mole = 60200000000000 In science, we deal with some very SMALL numbers: Mass of an electron = 0. 000000000000000091 kg

Scientific Notation Imagine the difficulty of calculating the mass of 1 mole of electrons! 0. 000000000000000091 kg x 60200000000000 ? ? ? ? ? ? ? ? ?

Scientific Notation: A method of representing very large or very small numbers in the form: M x 10 n Ø M is a number between 1 and 10 Ø n is an integer

2 500 000 . 9 8 7 6 5 4 3 2 1 Step #1: Insert an understood decimal point Step #2: Decide where the decimal must end up so that one number is to its left Step #3: Count how many places you bounce the decimal point Step #4: Re-write in the form M x 10 n

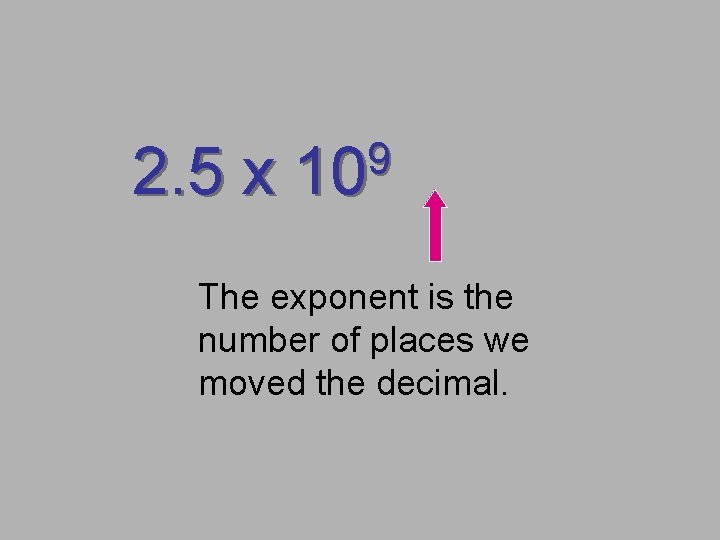
2. 5 x 9 10 The exponent is the number of places we moved the decimal.

0. 0000579 1 2 3 4 5 Step #2: Decide where the decimal must end up so that one number is to its left Step #3: Count how many places you bounce the decimal point Step #4: Re-write in the form M x 10 n

5. 79 x -5 10 The exponent is negative because the number we started with was less than 1.

PERFORMING CALCULATIONS IN SCIENTIFIC NOTATION ADDITION AND SUBTRACTION

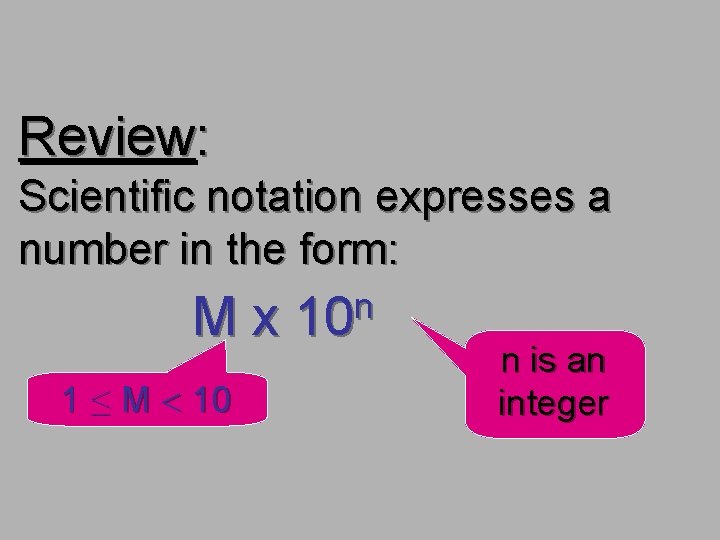
Review: Scientific notation expresses a number in the form: Mx 1 ≤ M < 10 n is an integer

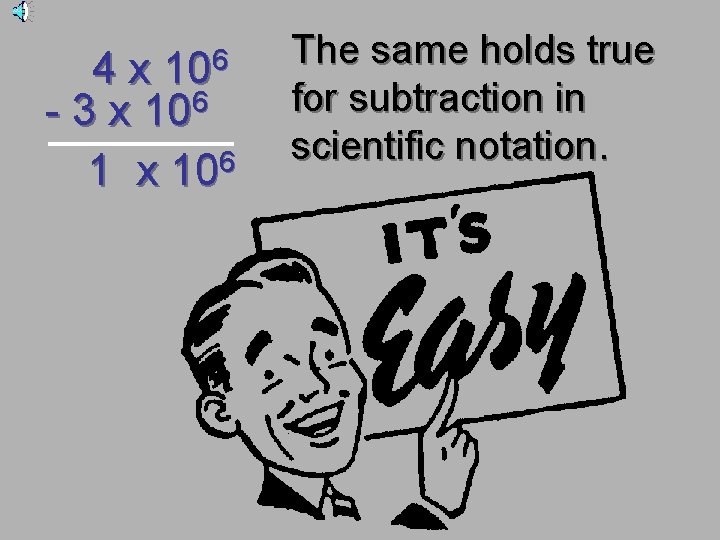
4 x 106 + 3 x 106 7 x 106 IF the exponents are the same, we simply add or subtract the numbers in front and bring the exponent down unchanged.

- 4 x 106 3 x 106 1 x 106 The same holds true for subtraction in scientific notation.

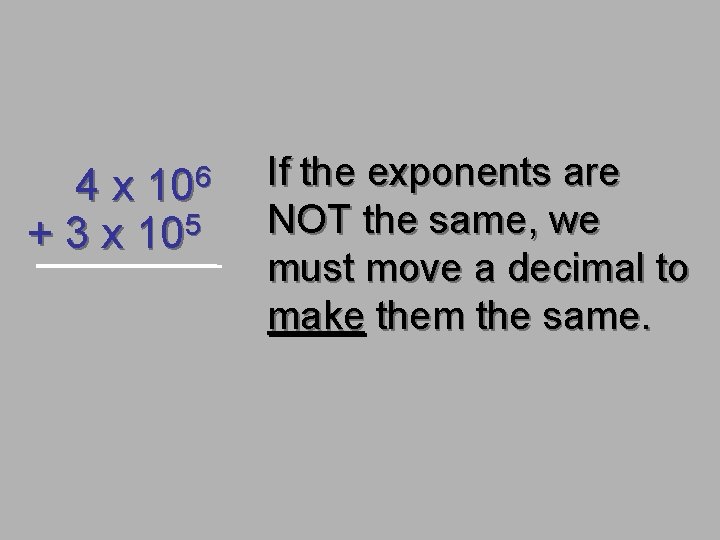
4 x 106 + 3 x 105 If the exponents are NOT the same, we must move a decimal to make them the same.

6 10 4. 00 x 5 + 3. 00 x 10 Move the decimal on the smaller number! 6 10 4. 00 x 6 +. 30 x 10 6 4. 30 x 10

A Problem for you… -6 10 2. 37 x -4 + 3. 48 x 10
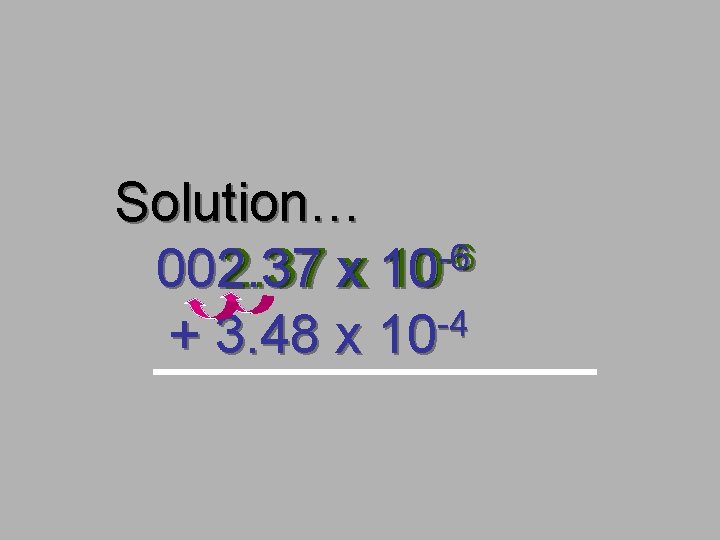
Solution… -6 -6 002. 37 xx 10 10 -4 + 3. 48 x 10
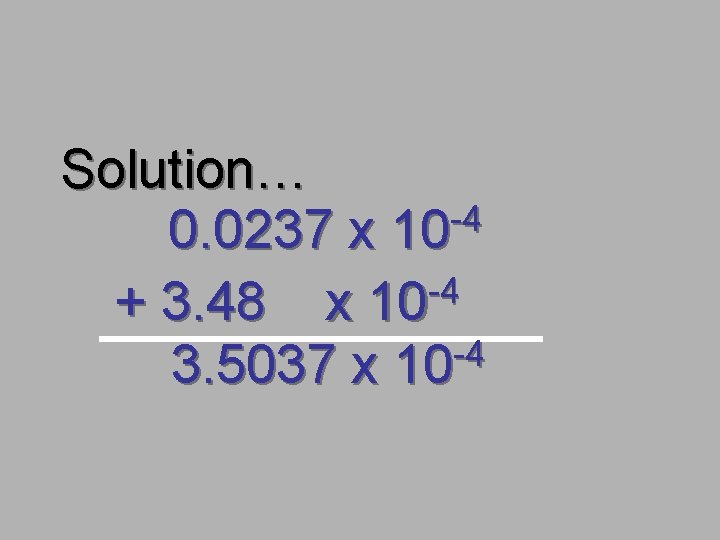
Solution… -4 0. 0237 x 10 -4 + 3. 48 x 10 -4 3. 5037 x 10

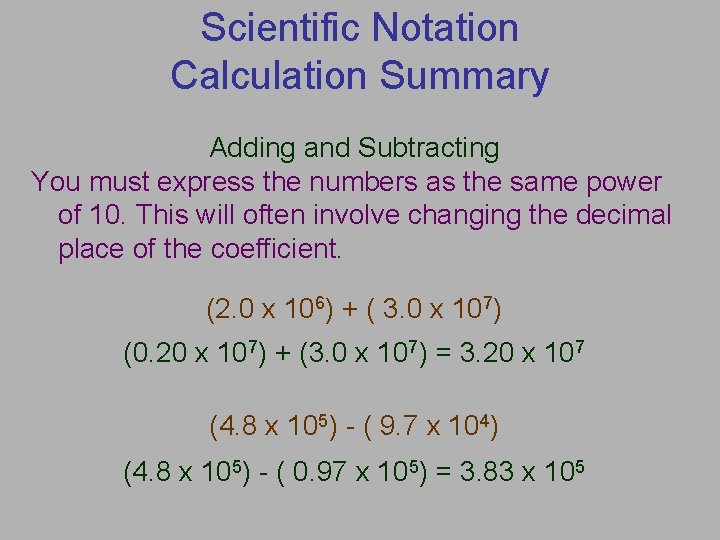
Scientific Notation Calculation Summary Adding and Subtracting You must express the numbers as the same power of 10. This will often involve changing the decimal place of the coefficient. (2. 0 x 106) + ( 3. 0 x 107) (0. 20 x 107) + (3. 0 x 107) = 3. 20 x 107 (4. 8 x 105) - ( 9. 7 x 104) (4. 8 x 105) - ( 0. 97 x 105) = 3. 83 x 105

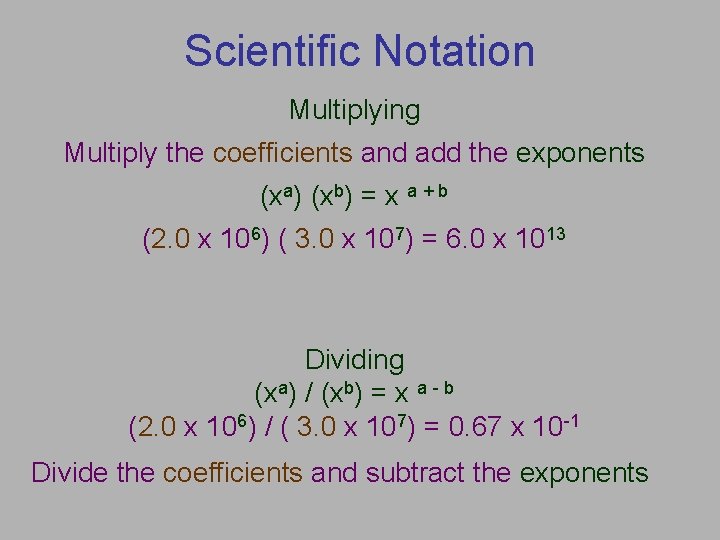
Scientific Notation Multiplying Multiply the coefficients and add the exponents (xa) (xb) = x a + b (2. 0 x 106) ( 3. 0 x 107) = 6. 0 x 1013 Dividing (xa) / (xb) = x a - b (2. 0 x 106) / ( 3. 0 x 107) = 0. 67 x 10 -1 Divide the coefficients and subtract the exponents

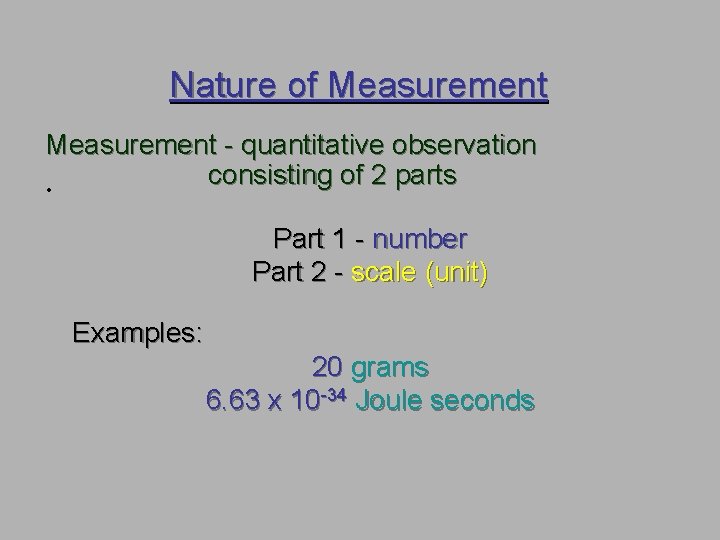
Nature of Measurement - quantitative observation consisting of 2 parts • Part 1 - number Part 2 - scale (unit) Examples: 20 grams 6. 63 x 10 -34 Joule seconds

Uncertainty in Measurement A digit that must be estimated is called uncertain. A measurement always has some degree of uncertainty.

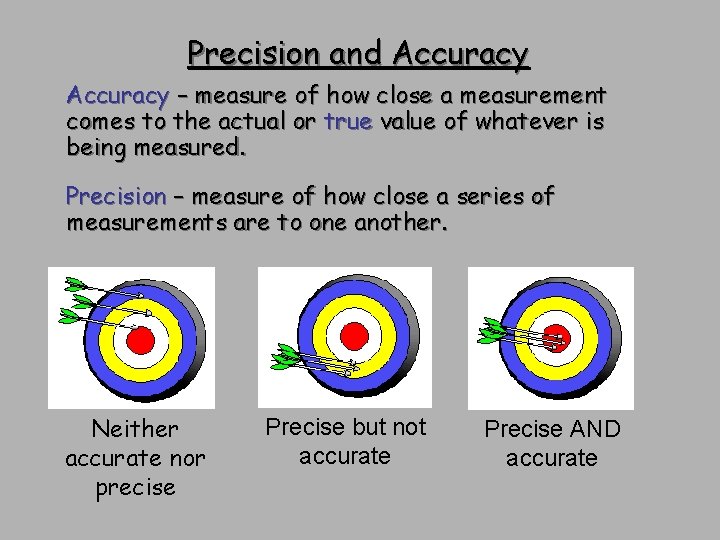
Precision and Accuracy – measure of how close a measurement comes to the actual or true value of whatever is being measured. Precision – measure of how close a series of measurements are to one another. Neither accurate nor precise Precise but not accurate Precise AND accurate

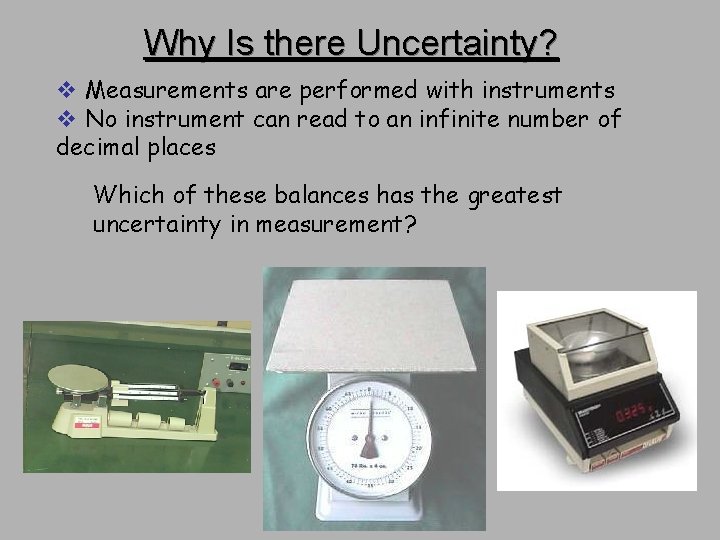
Why Is there Uncertainty? v Measurements are performed with instruments v No instrument can read to an infinite number of decimal places Which of these balances has the greatest uncertainty in measurement?

Determining Error Accepted Value – the correct value based on reliable references Experimental Value – the value measured in the lab Error(can be +or-)=experimental value – accepted value Percent error = absolute value of error x 100% accepted value

Significant Figures in Measurement In a supermarket, you can use the scales to measure the weight of produce. If you use a scale that is calibrated in 0. 1 lb intervals, you can easily read the scale to the nearest tenth of a pound. You can also estimate the weight to the nearest hundredth of a pound by noting the position of the pointer between calibration marks.

Significant Figures in Measurement If you estimate a weight that lies between 2. 4 lbs and 2. 5 lbs to be 2. 46 lbs, the number in this estimated measurement has three digits. The first two digits (2 and 4 ) are known with certainty. The rightmost digit (6) has been estimated and involves some uncertainty.

Significant Figures in Measurement Significant figures in a measurement include all of the digits that are know, plus a last digit that is estimated. Measurements must always be reported to the correct number of significant figures because calculated answers often depend on the number of significant figures in the values used in the calculation.

Rules for Counting Significant Figures Nonzero integers always count as significant figures. 3456 has 4 sig figs.

Rules for Counting Significant Figures Leading zeros do not count as significant figures. 0. 0486 has 3 sig figs.

Rules for Counting Significant Figures Zeros at the end of a number and to the right of a decimal point are always significant. 9. 000 has 4 sig figs 1. 010 has 4 sig figs

Rules for Counting Significant Figures Captive zeros always count as significant figures. 16. 07 has 4 sig figs.

Rules for Counting Significant Figures Zeros at the rightmost end that lie at the left of an understood decimal point are not significant. 7000 has 1 sig fig 27210 has 4 sig figs

Rules for Counting Significant Figures Exact numbers have an infinite number of significant figures. 1 inch = 2. 54 cm, exactly

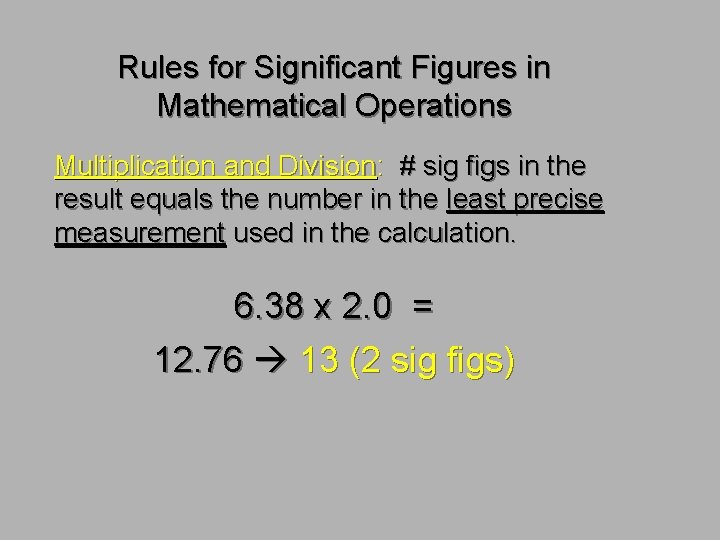
Rules for Significant Figures in Mathematical Operations Multiplication and Division: # sig figs in the result equals the number in the least precise measurement used in the calculation. 6. 38 x 2. 0 = 12. 76 13 (2 sig figs)

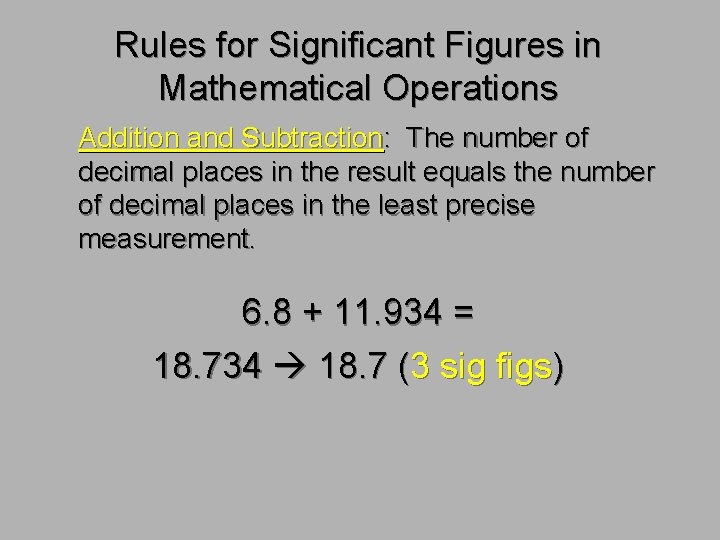
Rules for Significant Figures in Mathematical Operations Addition and Subtraction: The number of decimal places in the result equals the number of decimal places in the least precise measurement. 6. 8 + 11. 934 = 18. 734 18. 7 (3 sig figs)

Sig Fig Practice #1 How many significant figures in each of the following? 1. 0070 m 5 sig figs 17. 10 kg 4 sig figs 100, 890 L 5 sig figs 3. 29 x 103 s 3 sig figs 0. 0054 cm 2 sig figs 3, 200, 000 2 sig figs

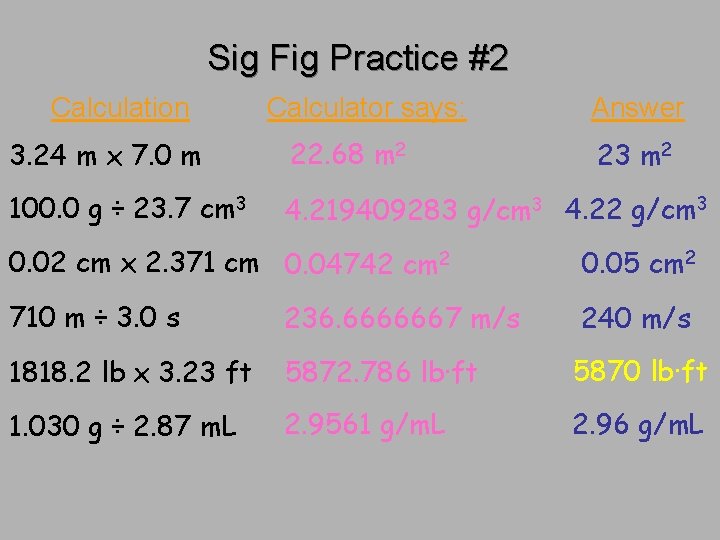
Sig Fig Practice #2 Calculation Calculator says: Answer 3. 24 m x 7. 0 m 22. 68 m 2 100. 0 g ÷ 23. 7 cm 3 4. 219409283 g/cm 3 4. 22 g/cm 3 23 m 2 0. 02 cm x 2. 371 cm 0. 04742 cm 2 0. 05 cm 2 710 m ÷ 3. 0 s 236. 6666667 m/s 240 m/s 1818. 2 lb x 3. 23 ft 5872. 786 lb·ft 5870 lb·ft 1. 030 g ÷ 2. 87 m. L 2. 9561 g/m. L 2. 96 g/m. L

Sig Fig Practice #3 Calculation Calculator says: Answer 3. 24 m + 7. 0 m 10. 24 m 10. 2 m 100. 0 g - 23. 73 g 76. 27 g 76. 3 g 0. 02 cm + 2. 371 cm 2. 39 cm 713. 1 L - 3. 872 L 709. 228 L 709. 2 L 1818. 2 lb + 3. 37 lb 1821. 57 lb 1821. 6 lb 2. 030 m. L - 1. 870 m. L 0. 160 m. L

Questions 1) 78ºC, 76ºC, 75ºC 2) 77ºC, 78ºC 2) 80ºC, 81ºC, 82ºC The sets of measurements were made of the boiling point of a liquid under similar conditions. Which set is the most precise? Set 2 – the three measurements are closest together. What would have to be known to determine which set is the most accurate? The accepted value of the liquid’s boiling point

Questions How do measurements relate to experimental science? Making correct measurements is fundamental to the experimental sciences. How are accuracy and precision evaluated? Accuracy is the measured value compared to the correct values. Precision is comparing more than one measurement.

Questions Why must a given measurement always be reported to the correct number of significant figures? The significant figures in a calculated answer often depend on the number of significant figures of the measurements used in the calculation. How does the precision of a calculated answer compare to the precision of the measurements used to obtain it? A calculated answer cannot be more precise than the least precise measurement used in the calculation.

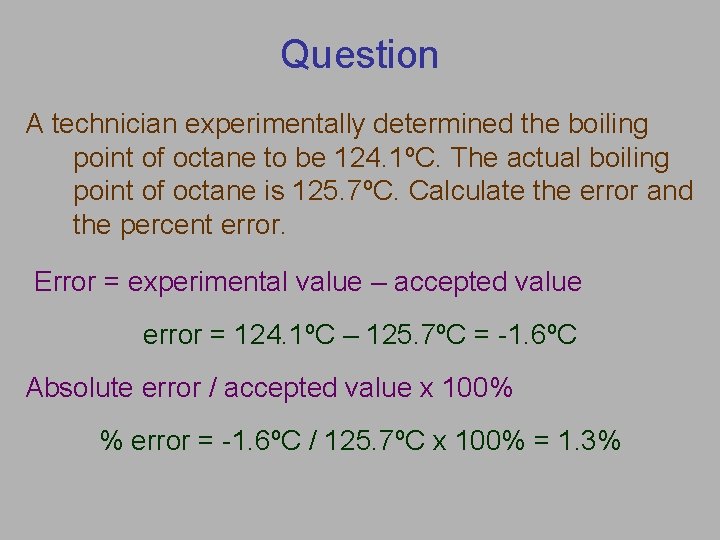
Question A technician experimentally determined the boiling point of octane to be 124. 1ºC. The actual boiling point of octane is 125. 7ºC. Calculate the error and the percent error. Error = experimental value – accepted value error = 124. 1ºC – 125. 7ºC = -1. 6ºC Absolute error / accepted value x 100% % error = -1. 6ºC / 125. 7ºC x 100% = 1. 3%

Question Determine the number of significant figures in each of the following 11 soccer player unlimited 0. 070 020 meter 5 10, 800 meters 3 5. 00 cubic meters 3

Question Solve the following and express each answer in scientific notation and to the correct number of significant figures. (5. 3 x 104) + (1. 3 x 104) 6. 6 x 104 (7. 2 x 10 -4) / (1. 8 x 103) 4. 0 x 10 -7 (104)(10 -3) (106) 107 (9. 12 x 10 -1) - (4. 7 x 10 -2) 8. 65 x 10 -1 (5. 4 x 104) (3. 5 x 109) 18. 9 x 1013 or 1. 9 x 10 14

International Systems of Units • The standards of measurement used in science are those of the metric system • All metric units are based on multiples of 10 • Metric system was originally establish in France in 1795 • The International System of Units (SI) is a revised version of the metric system. • The SI comes from the French name, le Systeme International d’Unites. • The SI was adopted by international agreement in 1960.

International Systems of Units There are seven SI base units SI Base Units SI base unit Symbol Meter m kilogram kg kelvin K second s mole mol Luminous intensity candela cd Electric current ampere A Quantity Length Mass Temperature Time Amount

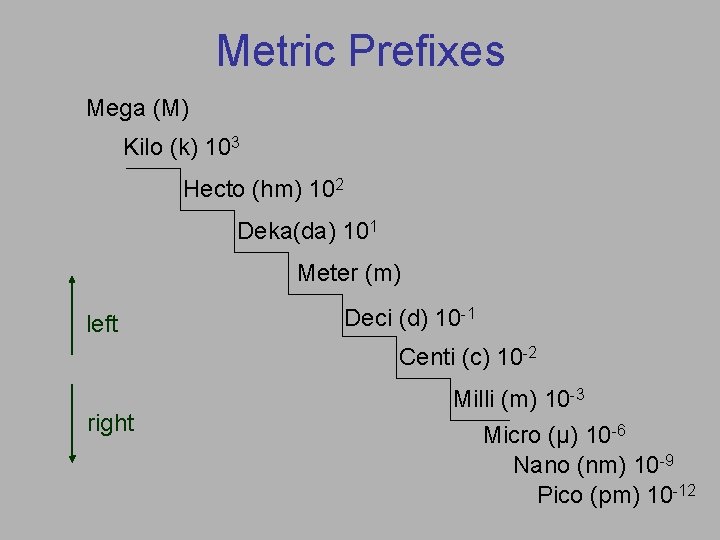
Metric Prefixes Mega (M) Kilo (k) 103 Hecto (hm) 102 Deka(da) 101 Meter (m) left Deci (d) 10 -1 Centi (c) 10 -2 right Milli (m) 10 -3 Micro (µ) 10 -6 Nano (nm) 10 -9 Pico (pm) 10 -12

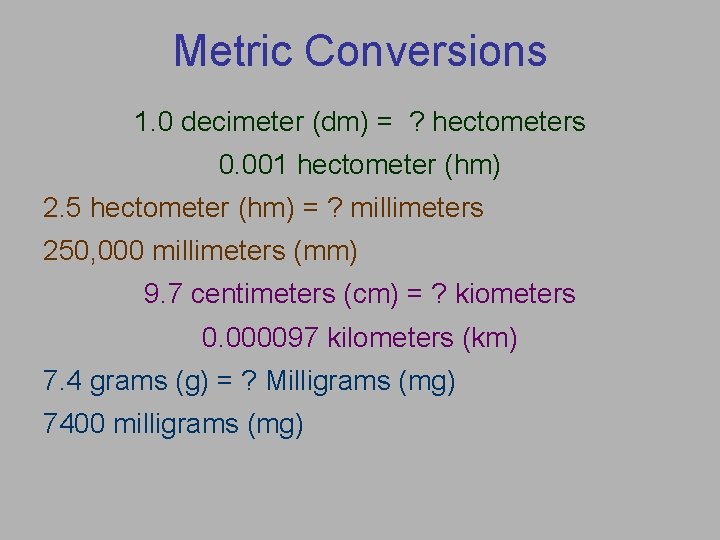
Metric Conversions 1. 0 decimeter (dm) = ? hectometers 0. 001 hectometer (hm) 2. 5 hectometer (hm) = ? millimeters 250, 000 millimeters (mm) 9. 7 centimeters (cm) = ? kiometers 0. 000097 kilometers (km) 7. 4 grams (g) = ? Milligrams (mg) 7400 milligrams (mg)

Other Common Conversions 1 cm 3 = 1 ml 1 dm 3 = 1 L 1 inch = 2. 54 cm 1 kg = 2. 21 lb 454 g = 1 lb 4. 18 J = 1 cal 1 mol = 6. 02 x 1023 pieces 1 GA = 3. 79 L

Units of Length meter – the basic SI unit of length or linear measure Common metric units of length include the centimeter (cm), meter (m), and kilometer (km)

Units of Volume -the space occupied by any sample of matter Volume (cube or rectangle) = length x width x height The SI unit of volume is the amount of space occupied by a cube that is 1 m along each edge. (m 3) Liter (L) – non SI unit – the volume of a cube that is 10 cm along each edge (1000 cm 3) The units milliliter and cubic centimeter are used interchangeably. 1 cm 3 = 1 ml 1 dm 3 = 1 L

Units of Mass Common metric units of mass include the kilogram, milligram and microgram. Weight – is a force that measures the pull on a given mass by gravity. Weight is a measure of force and is different than mass. Mass – measure of the quantity of matter. Although, the weight of an object can change with its location, its mass remains constant regardless of its location. Objects can become weightless, but not massless

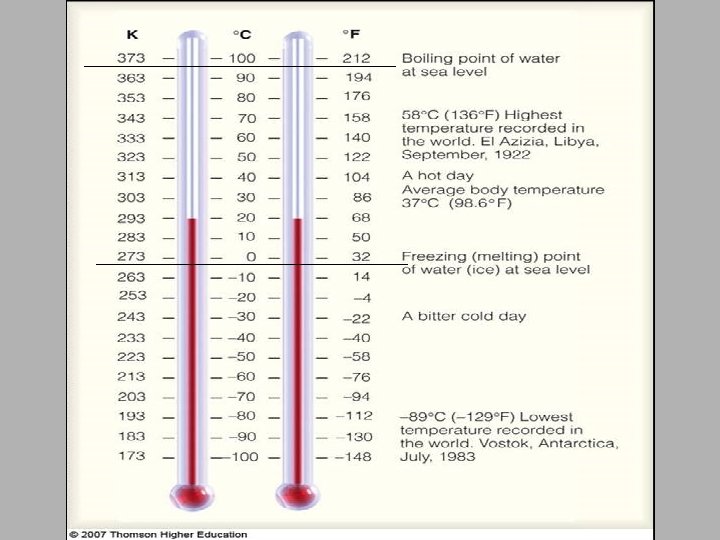
Units of Temperature – measure of how hot or cold an object is. The objects temperature determines the direction of heat transfer. When two objects at different temperatures are in contact, heat moves from the object at the higher temperature to the object at the lower temperature. Scientist use two equivalent units of temperature, the degree Celsius and the Kelvin.

Units of Temperature The Celsius scale of the metric system is named after Swedish astronomer Anders Celsius. The Celsius scale sets the freezing point of water at 0ºC and the boiling point of water at 100ºC The Kelvin scale is named for Lord Kelvin, a Scottish physicist and mathematician. On the Kelvin scale, the freezing point of water is 273. 15 kelvins (K), & the boiling point is 373. 15 K. With the Kelvin scale the degree (º) sign is not used

Units of Temperature A change of 1 º on the Celsius scale is equivalent to one kelvin on the Kelvin scale. The zero point on the Kelvin scale, 0 K, or absolute zero, is equal to -273. 15º C. K = ºC + 273 ºC = K - 273.


Units of Energy – the capacity to do work or to produce heat. The joule and the calorie are common units of energy. The joule (J) is the SI unit of energy named after the English physicist James Prescott Joule. 1 calorie (cal) - is the quanity of heat that raises the temperature of 1 g of pure water by 1ºC. 1 J = 0. 2390 cal 1 cal = 4. 184 J

Conversion Factors 1 dollar = 4 quarters = 10 dimes = 20 nickels = 100 pennies Different ways to express the same amount of money 1 meter =10 decimeters =100 centimeters =1000 millimeters Different ways to express length Whenever two measurements are equivalent, a ratio of the two measurements will equal 1. 1 m = 100 cm = 1 1 m Conversion factor

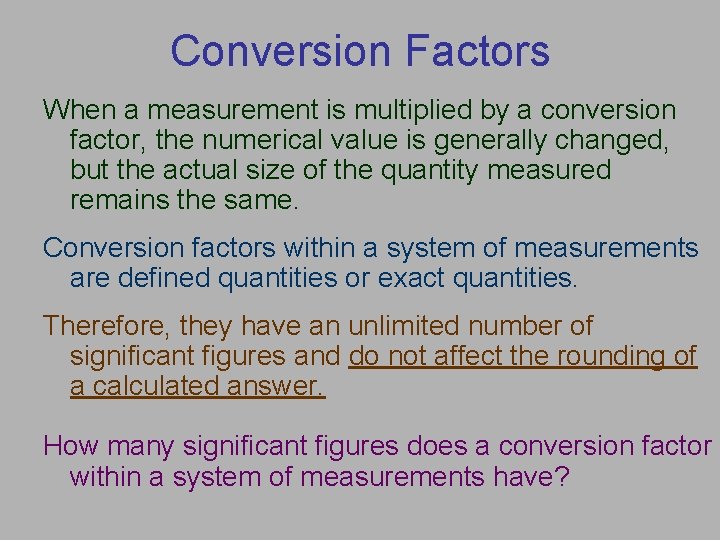
Conversion Factors Conversion factor – a ratio of equivalent measurements 100 cm / 1 m 1000 mm / 1 m The measurement on the top is equivalent to the measurement on the bottom Read “one hundred centimeters per meter” and “ 1000 millimeters per meter” Smaller number Larger number 1 m 100 cm larger unit smaller unit

Conversion Factors When a measurement is multiplied by a conversion factor, the numerical value is generally changed, but the actual size of the quantity measured remains the same. Conversion factors within a system of measurements are defined quantities or exact quantities. Therefore, they have an unlimited number of significant figures and do not affect the rounding of a calculated answer. How many significant figures does a conversion factor within a system of measurements have?

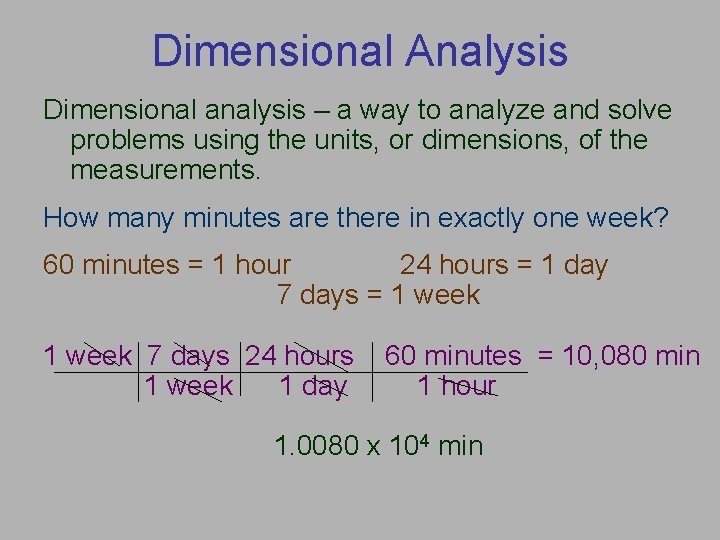
Dimensional Analysis Dimensional analysis – a way to analyze and solve problems using the units, or dimensions, of the measurements. How many minutes are there in exactly one week? 60 minutes = 1 hour 24 hours = 1 day 7 days = 1 week 7 days 24 hours 1 week 1 day 60 minutes = 10, 080 min 1 hour 1. 0080 x 104 min

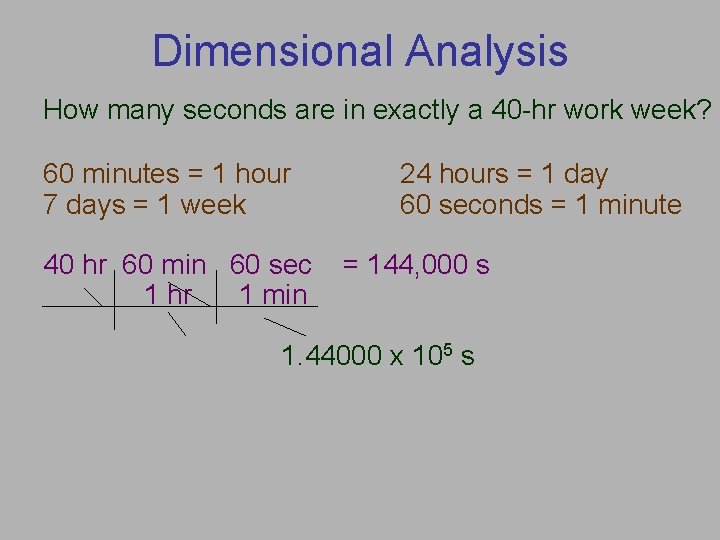
Dimensional Analysis How many seconds are in exactly a 40 -hr work week? 60 minutes = 1 hour 7 days = 1 week 40 hr 60 min 60 sec 1 hr 1 min 24 hours = 1 day 60 seconds = 1 minute = 144, 000 s 1. 44000 x 105 s

Dimensional Analysis An experiment requires that each student use an 8. 5 cm length of Mg ribbon. How many students can do the experiment if there is a 570 cm length of Mg ribbon available? 570 cm ribbon 2 sig figs 1 student 8. 5 cm ribbon = 67 students

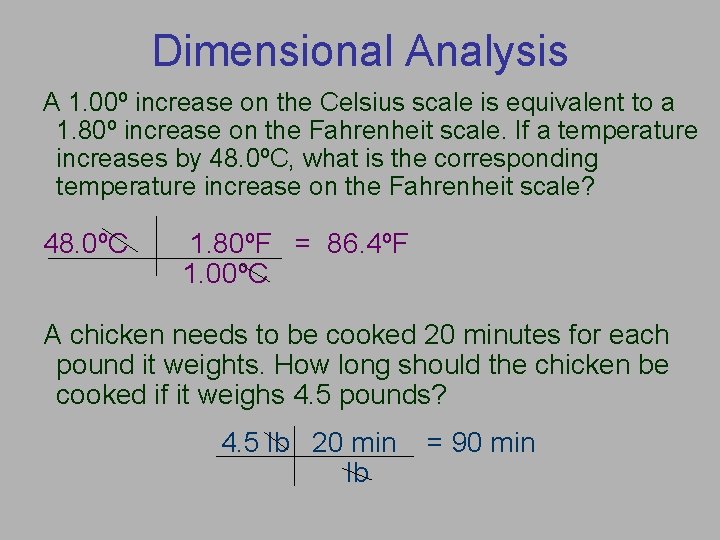
Dimensional Analysis A 1. 00º increase on the Celsius scale is equivalent to a 1. 80º increase on the Fahrenheit scale. If a temperature increases by 48. 0ºC, what is the corresponding temperature increase on the Fahrenheit scale? 48. 0ºC 1. 80ºF = 86. 4ºF 1. 00ºC A chicken needs to be cooked 20 minutes for each pound it weights. How long should the chicken be cooked if it weighs 4. 5 pounds? 4. 5 lb 20 min lb = 90 min

Dimensional Analysis Gold has a density of 19. 3 g/cm 3. What is the density in kg/m 3 19. 3 g 1 kg cm 3 1000 g 1 x 106 cm 3 = 1. 93 x 104 kg / m 3 There are 7. 0 x 106 red blood cell (RBC) in 1. 0 mm 3 of blood. How many red blood cells are in 1. 0 L of blood? 7. 0 x 106 RBC 1. 0 mm 3 1 x 106 mm 3 1 dm 3 = 7. 0 x 1012 dm 3 1 L

Dimensional Analysis 1. 00 L of neon gas contains 2. 69 x 1022 neon atoms. How many neon atoms are in 1. 00 mm 3 of neon gas under the same conditions? 2. 69 x 1022 atoms 1. 00 L 1 L 1 dm 3 1 x 106 mm 3 2. 69 x 1016 atoms in 1. 00 mm 3 of gas

Questions What conversion factor would you use to convert between these pairs of units? Minutes to hours 1 hour / 60 minutes grams to milligrams 1000 mg / 1 g Cubic decimeters to milliliters 1000 ml / 1 dm 3

Questions An atom of gold has a mass of 3. 271 x 10 -22 g. How many atoms of gold are in 5. 00 g of gold? 1. 53 x 1022 atoms of gold Light travels at a speed of 3. 00 x 1010 cm/sec. What is the speed of light in km/hour? 1. 08 x 109 km/hr

Questions Convert the following. Express your answers in scientific notation. 7. 5 x 104 J to k. J 7. 5 x 101 k. J 3. 9 x 105 mg to dg 3. 9 x 103 dg 2. 21 x 10 -4 d. L to µL 2. 21 x 101µL

Questions Make the following conversions. Express your answers in standard exponential form. 14. 8 g to µg 1. 48 x 107 µg 3. 75 x 10 -3 kg to g 3. 72 g 66. 3 L to cm 3 6. 63 x 104 cm 3

Density If a piece of led and a feather of the same volume are weighted, the lead would have a greater mass than the feather. It would take a much larger volume of feather to equal the mass of a given volume of lead. Density = mass / volume D=m/v Mass is a extensive property (a property that depends on the size of the sample) Density is an intensive property (depends on the composition of a substance, not on the size of the sample)

Density A helium filled balloon rapidly rises to the ceiling when released. Whether a gas-filled balloon will sink or rise when released depends on how the density of the gas compares with the density of air. Helium is less dense than air, so a helium filled balloon rises.

Density and Temperature The volume of most substances increase as the temperature increases. The mass remains the same despite the temperature and volume changes. So if the volume changes with temperature while the mass remains constant, then the density must also change with temperature. The density of a substance generally decreases as its temperature increases. (water is the exception: ice floats because it is less dense than liquid water)

Questions A student finds a shiny piece of metal that she thinks is aluminum. In the lab, she determines that the metal has a volume of 245 cm 3 and a mass of 612 g. Was is the density? Is it aluminum? D = 612 g / 245 cm 3 = 2. 50 g/cm 3 D of aluminum is 2. 70 g/cm 3; no it is not aluminum A bar of silver has a mass of 68. 0 g and a volume of 6. 48 cm 3. What is the density? D = 68. 0 g / 6. 48 cm 3 = 10. 5 g/cm 3

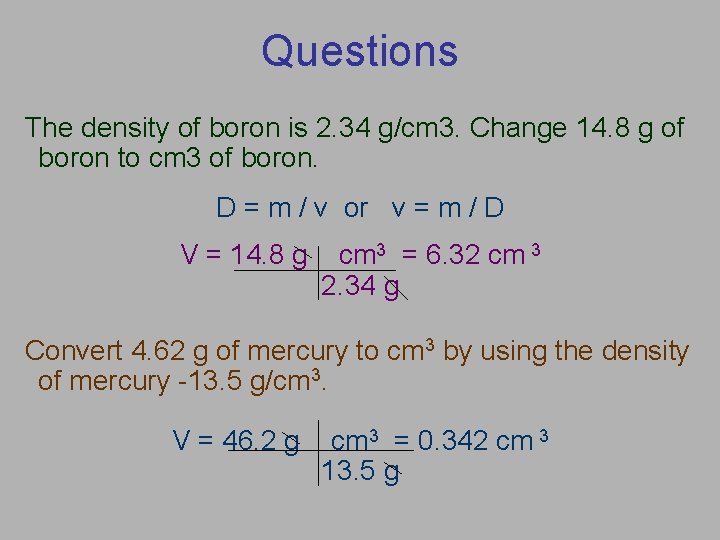
Questions The density of boron is 2. 34 g/cm 3. Change 14. 8 g of boron to cm 3 of boron. D = m / v or v = m / D V = 14. 8 g cm 3 = 6. 32 cm 3 2. 34 g Convert 4. 62 g of mercury to cm 3 by using the density of mercury -13. 5 g/cm 3. V = 46. 2 g cm 3 = 0. 342 cm 3 13. 5 g

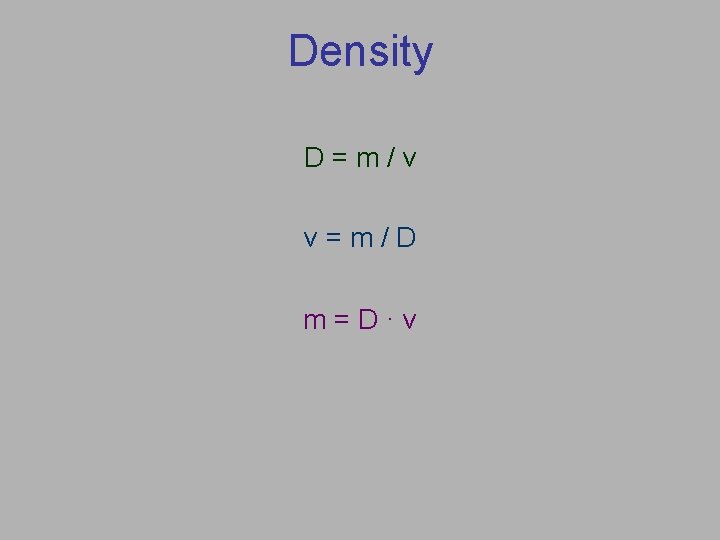
Density D=m/v v=m/D m=D·v