Chemistry Lesson 1 Ionic Compounds Ionic Bonds l
Chemistry Lesson # 1 Ionic Compounds
Ionic Bonds l Atoms gain or lose electrons when they are trying to combine, or bond, with other atoms, because electrons are being transferred between them. l When two or more atoms combine, the result is called a compound. l The atoms are attracted to each other because of their opposite charge. The reason this occurs is so that reactive elements become stable when combined. l Stable means that their outermost shell of electrons is completely full, usually with eight electrons.

Characteristics of Ionic Compounds l Compounds tend to be hard, brittle solids at room temperature. l They tend to form organized crystals with high melting points, which easily dissolve in water. l They can weakly conduct electricity. l This is why a grain of salt looks like a perfect cube, that it only melts at 800°C, and why we have salt water! l Another name for salt is sodium chloride, which is the ionic bond between sodium and chlorine.
How Sodium Bonds with Chlorine

How Magnesium Bonds with Fluorine

Ionic Charges l All elements have a certain ionic charge, which is also called the valence, or combining capacity. l Some only have one ionic charge possible, others have two or three. These elements combine by transferring electrons. l Metals LOSE electrons to form positive ions l Non-metals GAIN electrons to form negative ions l The result is an electrically neutral compound, where the sum of the charges on the positive ions equals the sum of the charges on the negative ions (charge is now zero).
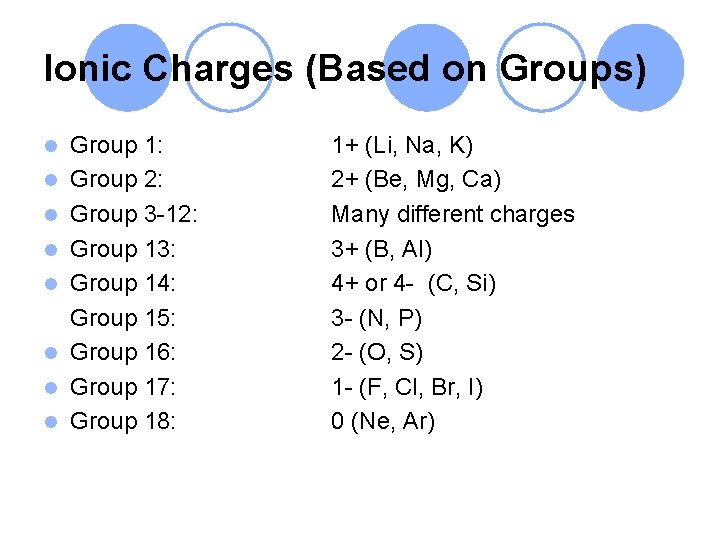
Ionic Charges (Based on Groups) l l l l Group 1: Group 2: Group 3 -12: Group 13: Group 14: Group 15: Group 16: Group 17: Group 18: 1+ (Li, Na, K) 2+ (Be, Mg, Ca) Many different charges 3+ (B, Al) 4+ or 4 - (C, Si) 3 - (N, P) 2 - (O, S) 1 - (F, Cl, Br, I) 0 (Ne, Ar)

Naming & Writing Formulas l Now that we know the charges for elements, we can use a simpler method to determine the formula of the compound. l I call it the “criss cross” rule. l To name compounds, the metal comes first, followed by the non-metal, with the ending changing to “ide” – example: sodium + chlorine makes “sodium chloride” l Anytime each of the charges are the same or multiples of one another, you may reduce the charges.
Ca + I Mg + O
Al + S H+P

Ionic Compounds with Multiple Charges l Metals with multiple charges will have a roman numeral in brackets which you use as the charge on that metal. Example – copper (II) bromide means that copper has a charge of 2+. Don’t forget – metals always have a positive charge. You can use this charge in the criss cross rule to determine the formula. l If you are given a formula with a transition metal, you must determine the charge used by reversing back the criss-cross rule and writing the roman numeral into the name. l Some transition metals have only one charge. In this case, a roman numeral should not be used. Some examples include silver (1+), zinc (2+), and aluminum (3+).
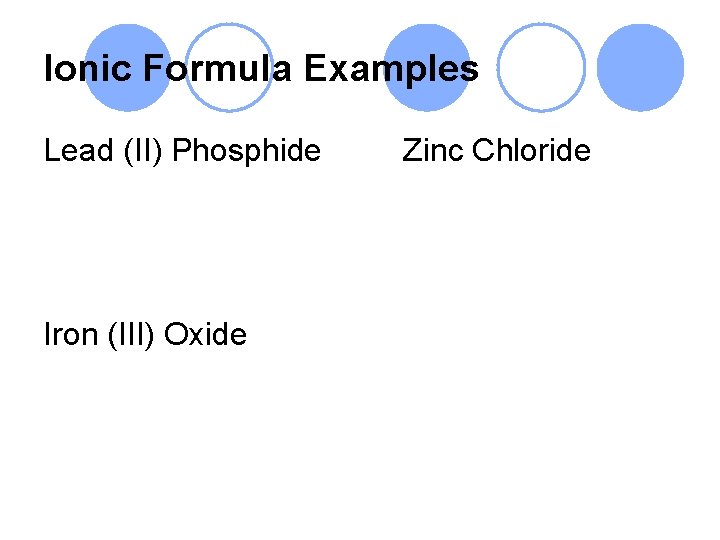
Ionic Formula Examples Lead (II) Phosphide Iron (III) Oxide Zinc Chloride

Ionic Naming Examples Ga 2 S 3 Al. P Cu. F 2
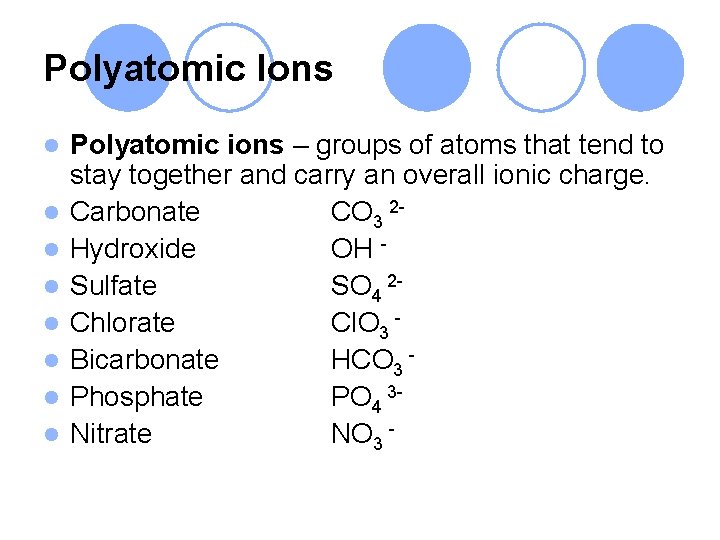
Polyatomic Ions l l l l Polyatomic ions – groups of atoms that tend to stay together and carry an overall ionic charge. Carbonate CO 3 2 Hydroxide OH Sulfate SO 4 2 Chlorate Cl. O 3 Bicarbonate HCO 3 Phosphate PO 4 3 Nitrate NO 3 -
Polyatomic Ions (continued) l These anions can combine with positive metals in the same way as before to form neutral polyatomic compounds. l Naming – metal first, followed by the unchanged name of polyatomic ion makes the full name (no changing the ending like in ionic). Example – sodium + chlorate makes “sodium chlorate” l A bracket should be put around the polyatomic compound to distinguish between the polyatomic numbers and the criss-cross numbers.
Polyatomic Examples Sodium + Sulfate Aluminum + Nitrate Magnesium + Hydroxide Lead (IV) + Carbonate
Naming Acids l Acids are ionic compounds where hydrogen is always the positive ion (cation). l Acids can involve anions (single nonmetals) or polyatomic compounds.

1. Hydrogen + Non-Metal l When hydrogen pairs with an anion, the word "hydro" starts the naming, and ends in "ic" instead of "ide. " l For example – hydrogen + chlorine makes hydrochloric acid. l These acids are also called binary acids, since they only have two parts.
Examples Hydrogen + Phosphorus Hydrogen + Bromine Hydrogen + Sulfur

2. Hydrogen + Polyatomic Ions l When hydrogen pairs with a polyatomic compound, they “hydro” is dropped. This is because the prefix usually indicates an absence of oxygen, and polyatomics all contain oxygen. l These acids are sometimes called oxyacids.
Examples Hydrogen + Chlorate Hydrogen + Carbonate Hydrogen + Nitrate

Acids - Working Backwards l Determine the formula for the following acids. Don’t forget that as soon as you see the word “acid”, you know you are starting with hydrogen. Sulfuric Acid Hydrophosphoric Acid

Videos l Quick l More Na. Cl clip detailed Na. Cl clip
- Slides: 23