Chemistry Joke of the Day Announcements n n











- Slides: 15

Chemistry Joke of the Day

Announcements n n n Exam #1: Tonight!, 7: 00 -8: 30 pm, locations posted on website, bring ID Conflict Exam: 5: 00 -6: 30 pm, 217 Noyes, bring ID No book homework due Friday! Lecture on Thursday! (New Material) Exam scores posted late Thursday evening. Get graded exams back on Friday. Liquid nitrogen ice cream winner = highest section average!!!

Exam Format – 90 minutes total 15 Multiple Choice Questions n 5 possible choices n 50% of test grade n ~3 minutes to answer each (~45 min on this half of exam) 2 Free Response Questions n n n Write in answers – both qualitative and quantitative 50% of test grade ~45 minutes on this half of exam ***A periodic table & cover sheet with equations will be attached. ***

Top Review Topics You Asked For Concentration of ions problems 2. Conceptual molarity problems 3. Stoichiometry involving gases 4. Neutralization 5. What to do if solubility rules aren’t covered Also… “STOICH!” 1.

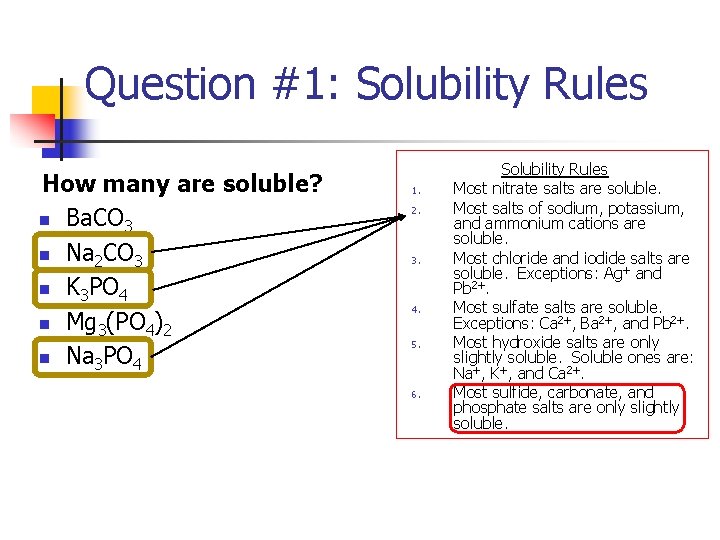
Question #1: Solubility Rules How many are soluble? n Ba. CO 3 n Na 2 CO 3 n K 3 PO 4 n Mg 3(PO 4)2 n Na 3 PO 4 1. 2. 3. 4. 5. 6. Solubility Rules Most nitrate salts are soluble. Most salts of sodium, potassium, and ammonium cations are soluble. Most chloride and iodide salts are soluble. Exceptions: Ag+ and Pb 2+. Most sulfate salts are soluble. Exceptions: Ca 2+, Ba 2+, and Pb 2+. Most hydroxide salts are only slightly soluble. Soluble ones are: Na+, K+, and Ca 2+. Most sulfide, carbonate, and phosphate salts are only slightly soluble.

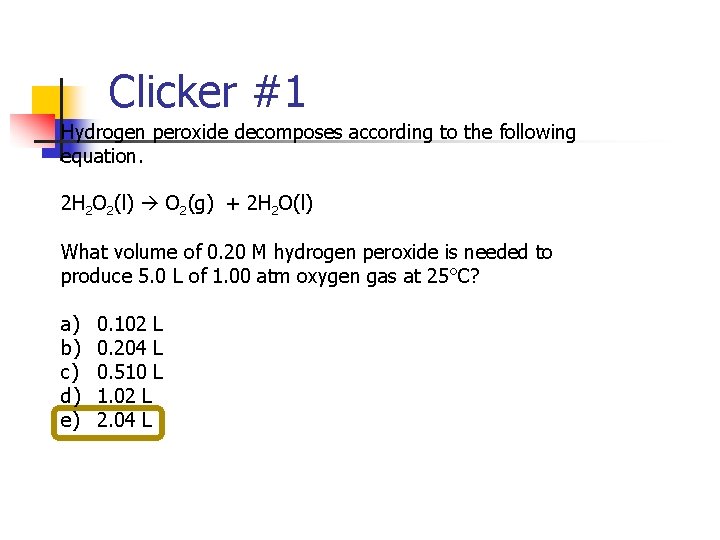
Clicker #1 Hydrogen peroxide decomposes according to the following equation. 2 H 2 O 2(l) O 2(g) + 2 H 2 O(l) What volume of 0. 20 M hydrogen peroxide is needed to produce 5. 0 L of 1. 00 atm oxygen gas at 25°C? a) b) c) d) e) 0. 102 L 0. 204 L 0. 510 L 1. 02 L 2. 04 L

Clicker #2 100. 0 m. L of a 0. 200 M Ba(OH)2 solution is neutralized with 0. 100 M HCl. What volume of HCl is needed? Ba(OH)2(aq) + 2 HCl(aq) 2 H 2 O(l) + Ba. Cl 2(aq) a) 50. 0 m. L b) 100. 0 m. L c) 200. 0 m. L d) 300. 0 m. L e) 400. 0 m. L

Clicker #3 Consider the reaction that occurs when 4. 0 L of 2. 00 M silver nitrate reacts with 2. 0 L of 3. 00 M sodium carbonate to form a white precipitate. 2 Ag. NO 3(aq) Na 2 CO 3(aq) Ag 2 CO 3(s) + 2 Na. NO 3(aq) 2 Ag+(aq) + CO 32 -(aq) Ag 2 CO 3(s) What is the concentration of sodium ions after the reaction? a) b) c) d) e) 0 M 1. 00 M 2. 00 M 3. 00 M 6. 00 M

Clicker #4 Consider the reaction that occurs when 4. 0 L of 2. 00 M silver nitrate reacts with 2. 0 L of 3. 00 M sodium carbonate to form a white precipitate. 2 Ag. NO 3(aq) Na 2 CO 3(aq) Ag 2 CO 3(s) + 2 Na. NO 3(aq) 2 Ag+(aq) + CO 32 -(aq) Ag 2 CO 3(s) What is the concentration of nitrate ions after the reaction? a) 0. 33 M b) 1. 00 M c) 1. 33 M d) 2. 00 M e) 4. 00 M

Clicker #5 Consider the reaction that occurs when 4. 0 L of 2. 00 M silver nitrate reacts with 2. 0 L of 3. 00 M sodium carbonate to form a white precipitate. 2 Ag. NO 3(aq) Na 2 CO 3(aq) Ag 2 CO 3(s) + 2 Na. NO 3(aq) 2 Ag+(aq) + CO 32 -(aq) Ag 2 CO 3(s) What is the concentration of silver ions after the reaction? a) 0 M b) 1. 33 M c) 1. 67 M d) 2. 00 M e) 4. 00 M

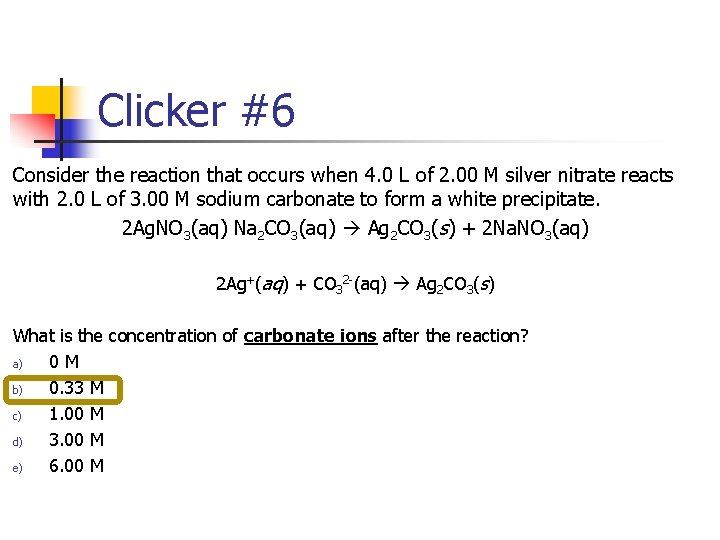
Clicker #6 Consider the reaction that occurs when 4. 0 L of 2. 00 M silver nitrate reacts with 2. 0 L of 3. 00 M sodium carbonate to form a white precipitate. 2 Ag. NO 3(aq) Na 2 CO 3(aq) Ag 2 CO 3(s) + 2 Na. NO 3(aq) 2 Ag+(aq) + CO 32 -(aq) Ag 2 CO 3(s) What is the concentration of carbonate ions after the reaction? a) 0 M b) 0. 33 M c) 1. 00 M d) 3. 00 M e) 6. 00 M

Clicker #7 Consider the equation: A + 3 B 4 C. If 3. 0 moles of A is reacted with 6. 0 moles of B, which of the following is true after the reaction is complete? a) A is the leftover reactant because you only need 2 moles of A and have 3. b) A is the leftover reactant because for every 1 mole of A, 4 moles of C are produced. c) B is the leftover reactant because you have more moles of B than A. d) B is the leftover reactant because 3 moles of B react with every 1 mole of A. e) Neither reactant is leftover.

Clicker #8 You pour Solution #1 and Solution #4 together in a large, empty beaker. What is the concentration of the new HCl mixture? a) 3. 00 M b) 3. 25 M c) 3. 40 M d) 4. 25 M e) 6. 50 M

Clicker #9 You have a 16. 0 oz (473 m. L) glass of lemonade with a concentration of 2. 00 M. The lemonade sits out on your counter for a couple of days and 150. m. L of water evaporates from the glass. What is the new concentration of the lemonade? a) 1. 52 M b) 2. 00 M c c) 2. 93 M d) 6. 31 M e)none of these

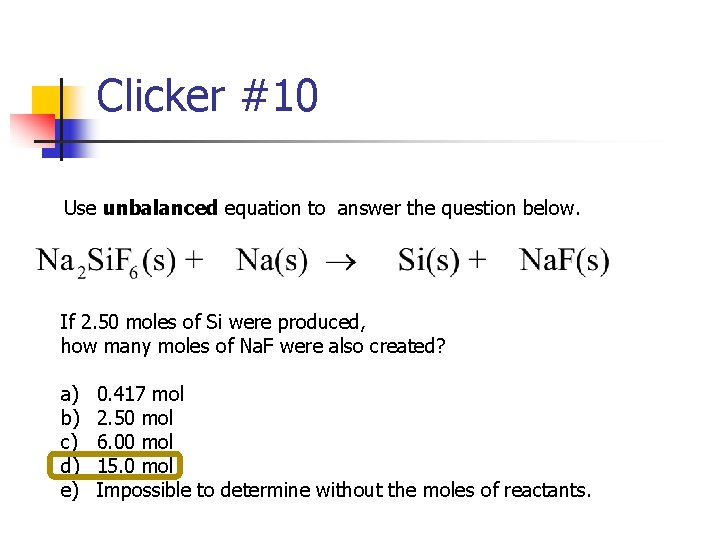
Clicker #10 Use unbalanced equation to answer the question below. If 2. 50 moles of Si were produced, how many moles of Na. F were also created? a) b) c) d) e) 0. 417 mol 2. 50 mol 6. 00 mol 15. 0 mol Impossible to determine without the moles of reactants.
 Day 1 day 2 day 3 day 4
Day 1 day 2 day 3 day 4 Day 1 day 2 day 817
Day 1 day 2 day 817 General announcements
General announcements David ritthaler
David ritthaler Fahrenheit 451 burning bright
Fahrenheit 451 burning bright Pvu market cap
Pvu market cap Potentiial
Potentiial R/announcements
R/announcements Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Biological macromolecules poem
Biological macromolecules poem Dirty panda joke eats shoots and leaves
Dirty panda joke eats shoots and leaves Reactant image
Reactant image Bs ms phd joke
Bs ms phd joke Inflation
Inflation Keitai bango
Keitai bango