Chemistry Ions Electron Configurations of Noble Gases Write

Chemistry Ions
Electron Configurations of Noble Gases �Write out the electron configuration for the following elements: �Neon �Argon

Noble Gases �Neon can also be written: (He) 2 s 2 2 p 6 �Argon can also be written: (Ne) 3 s 2 3 p 6 �Krypton can be written: (Ar) 3 d 10 4 s 2 4 p 6 �What do all of the above have in common?
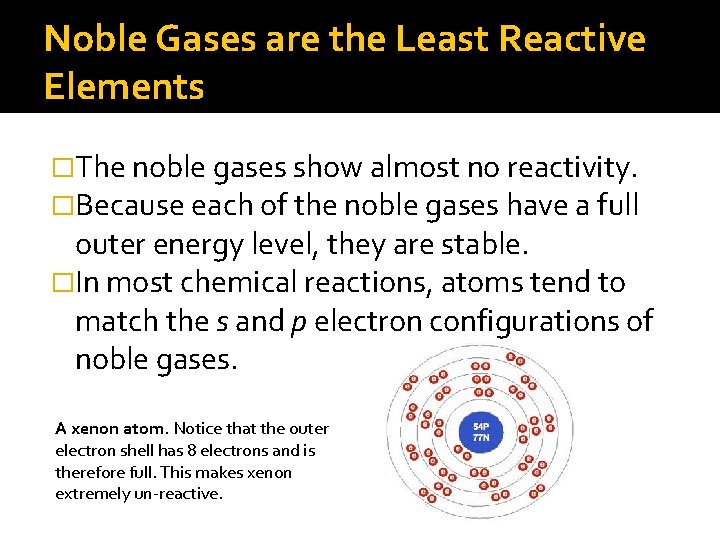
Noble Gases are the Least Reactive Elements �The noble gases show almost no reactivity. �Because each of the noble gases have a full outer energy level, they are stable. �In most chemical reactions, atoms tend to match the s and p electron configurations of noble gases. A xenon atom. Notice that the outer electron shell has 8 electrons and is therefore full. This makes xenon extremely un-reactive.
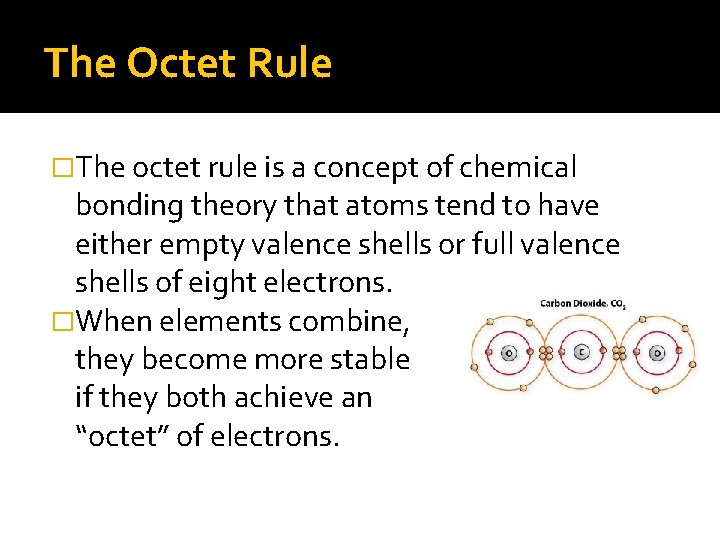
The Octet Rule �The octet rule is a concept of chemical bonding theory that atoms tend to have either empty valence shells or full valence shells of eight electrons. �When elements combine, they become more stable if they both achieve an “octet” of electrons.
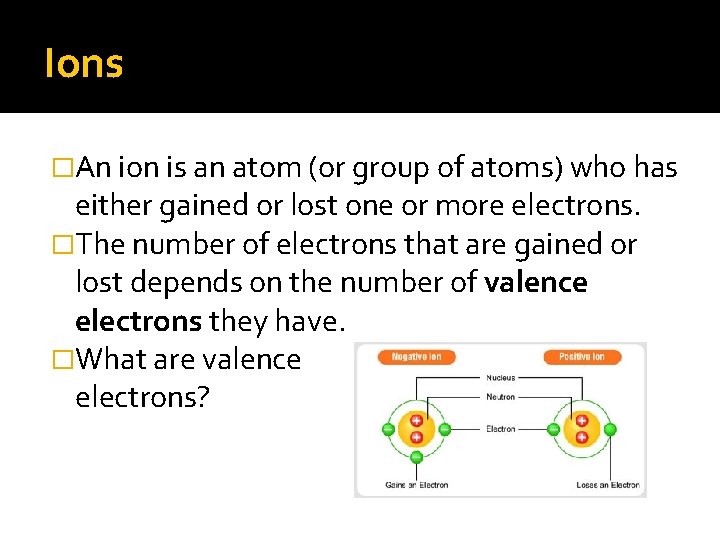
Ions �An ion is an atom (or group of atoms) who has either gained or lost one or more electrons. �The number of electrons that are gained or lost depends on the number of valence electrons they have. �What are valence electrons?
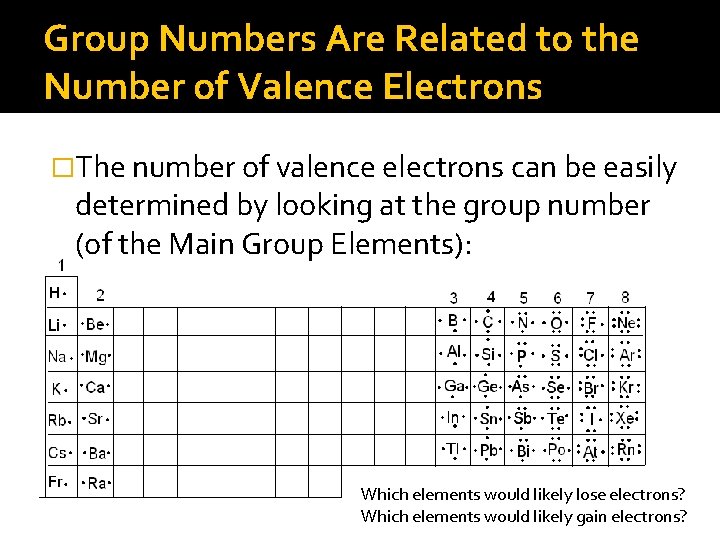
Group Numbers Are Related to the Number of Valence Electrons �The number of valence electrons can be easily determined by looking at the group number (of the Main Group Elements): Which elements would likely lose electrons? Which elements would likely gain electrons?
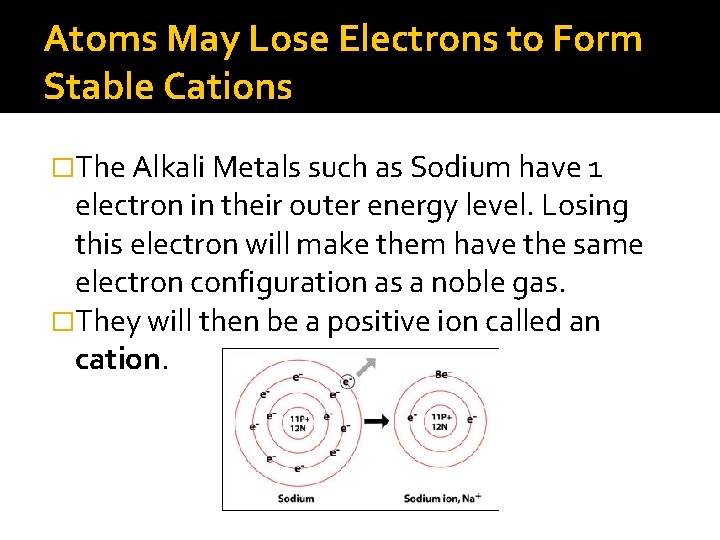
Atoms May Lose Electrons to Form Stable Cations �The Alkali Metals such as Sodium have 1 electron in their outer energy level. Losing this electron will make them have the same electron configuration as a noble gas. �They will then be a positive ion called an cation.
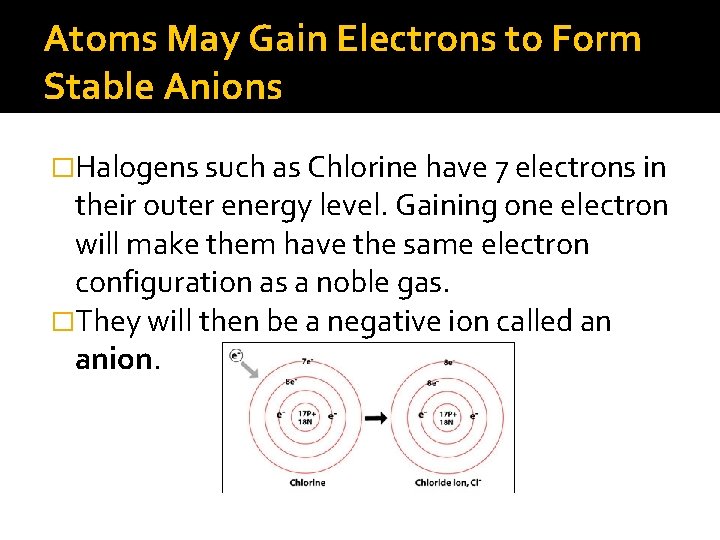
Atoms May Gain Electrons to Form Stable Anions �Halogens such as Chlorine have 7 electrons in their outer energy level. Gaining one electron will make them have the same electron configuration as a noble gas. �They will then be a negative ion called an anion.
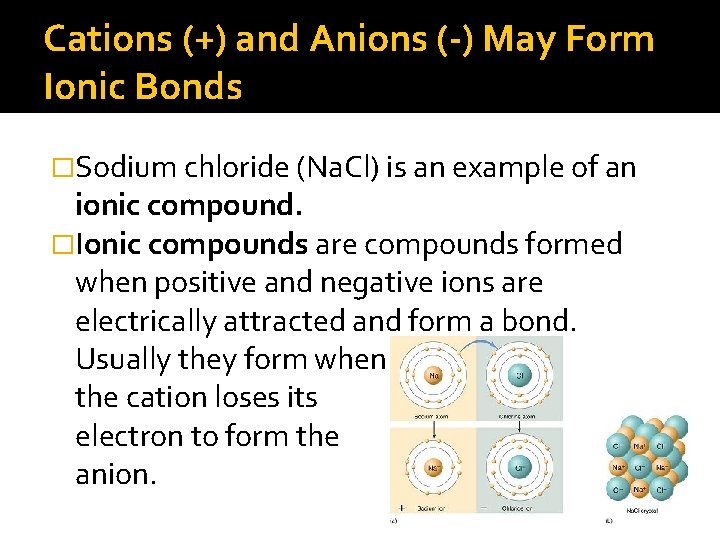
Cations (+) and Anions (-) May Form Ionic Bonds �Sodium chloride (Na. Cl) is an example of an ionic compound. �Ionic compounds are compounds formed when positive and negative ions are electrically attracted and form a bond. Usually they form when the cation loses its electron to form the anion.
- Slides: 10