CHEMISTRY IN BIOLOGY To understand life you need

CHEMISTRY IN BIOLOGY To understand life, you need to understand the important elements that make up living organisms

Basic building blocks of matter Make up everything Made of 3 parts Proton Neutron Electron ATOMS
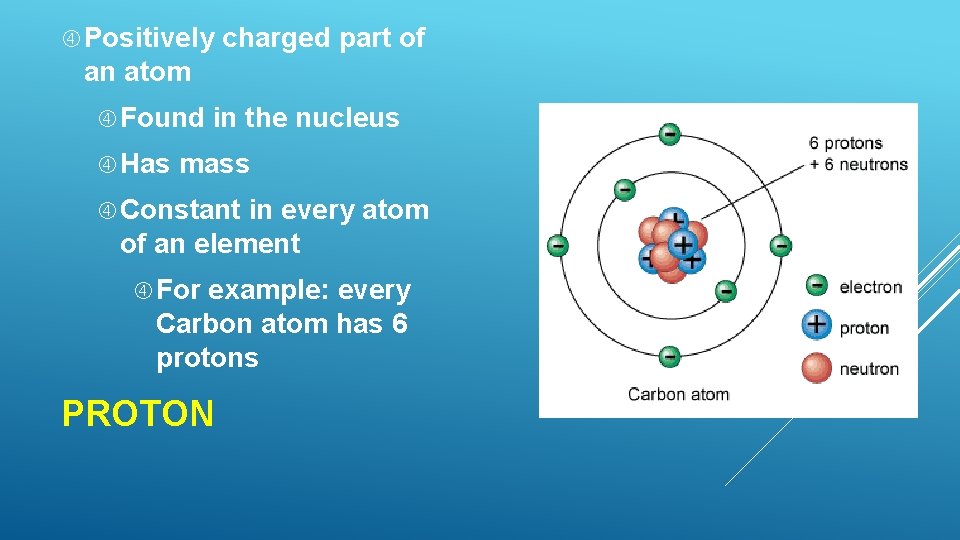
Positively charged part of an atom Found Has in the nucleus mass Constant in every atom of an element For example: every Carbon atom has 6 protons PROTON
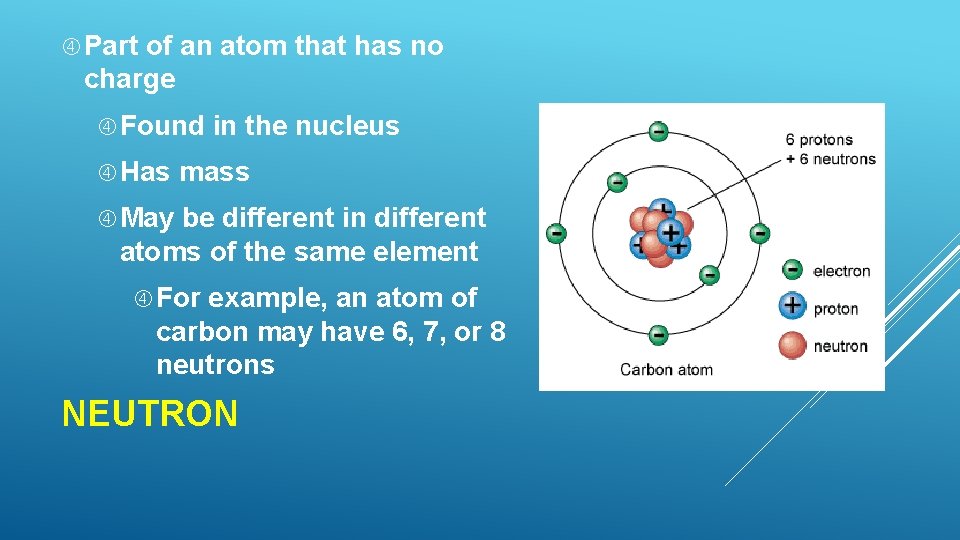
Part of an atom that has no charge Found Has in the nucleus mass May be different in different atoms of the same element For example, an atom of carbon may have 6, 7, or 8 neutrons NEUTRON
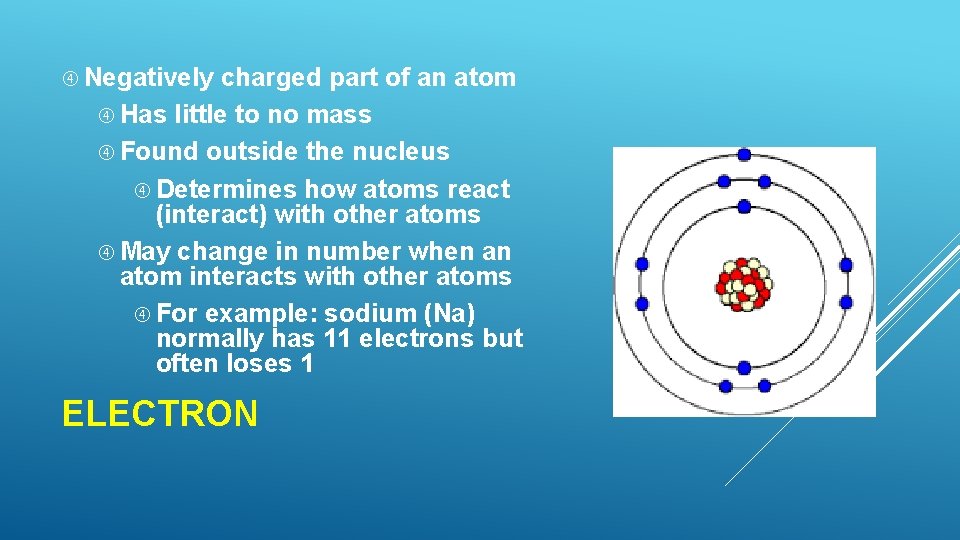
Negatively charged part of an atom Has little to no mass Found outside the nucleus Determines how atoms react (interact) with other atoms May change in number when an atom interacts with other atoms For example: sodium (Na) normally has 11 electrons but often loses 1 ELECTRON
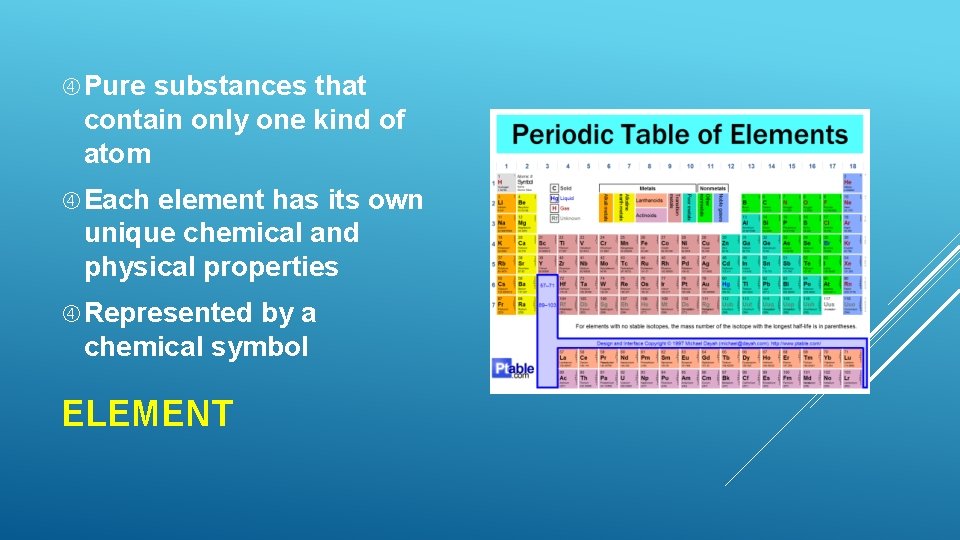
Pure substances that contain only one kind of atom Each element has its own unique chemical and physical properties Represented by a chemical symbol ELEMENT

Ø ALL ORGANISMS REQUIRE CARBON, HYDROGEN, OXYGEN, NITROGEN, AND PHOSPHORUS IN RELATIVELY LARGE AMOUNTS. THESE ELEMENTS PASS BETWEEN LIVING ORGANISMS AND NONLIVING ENVIRONMENTS IN CLOSED CYCLES
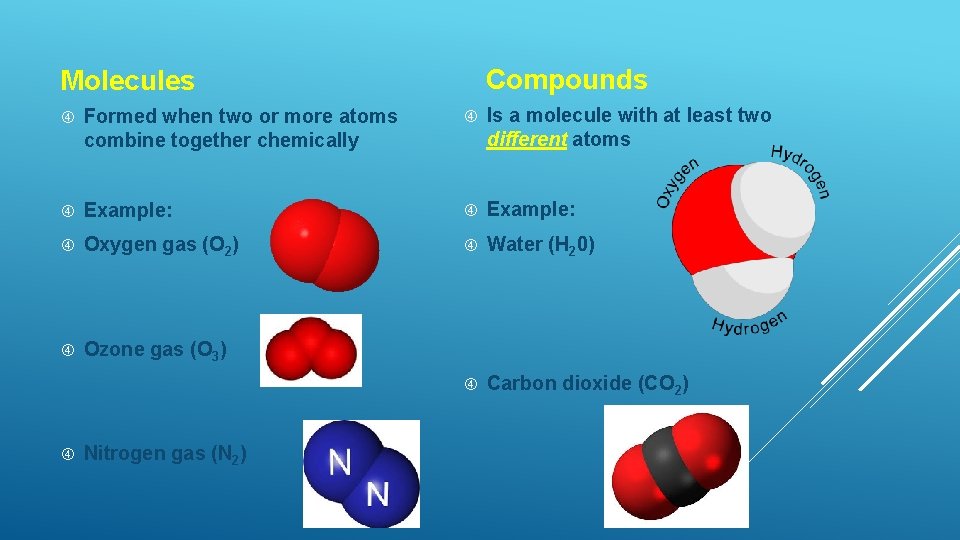
Compounds Molecules Formed when two or more atoms combine together chemically Is a molecule with at least two different atoms Example: Oxygen gas (O 2) Water (H 20) Ozone gas (O 3) Carbon dioxide (CO 2) Nitrogen gas (N 2)
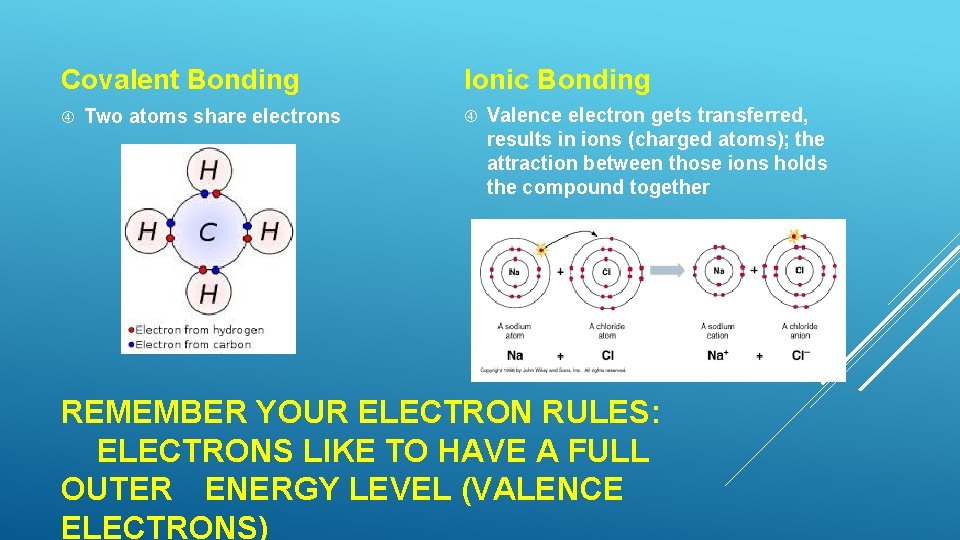
Covalent Bonding Two atoms share electrons Ionic Bonding Valence electron gets transferred, results in ions (charged atoms); the attraction between those ions holds the compound together REMEMBER YOUR ELECTRON RULES: ELECTRONS LIKE TO HAVE A FULL OUTER ENERGY LEVEL (VALENCE ELECTRONS)
- Slides: 9