Chemistry IA Chapter 4 ATOMIC STRUCTURE 4 1





















- Slides: 40

Chemistry IA Chapter 4 ATOMIC STRUCTURE

4. 1 Studying Atoms n Ancient Greek Models of Atoms n Demokritos n n Believed matter was composed of very small particles that he called ‘atomos’ which means ‘indivisible’ in Greek. Thought different types of atoms had different properties. § Atoms of liquids were round & smooth. § Atoms of solids were rough & prickly.

Aristotle & Prime Matter n Aristotle n n Believed matter was infinitely small or could be any size. Believed in Four forms of matter he called ‘Prime Matter’: § § n n Fire Earth Air Water Combining of any of these could produce anything. See p. 100, Figure 1

Dalton’s Atomic Theory n n English Science teacher who studied gases for fun. Found compounds had ‘fixed’ compositions. n n n Example: When Magnesium burns, it forms a new compound that contains oxygen combined with magnesium. He found that no matter how much you magnesium you start with, 65. 8% of the new compound will be oxygen and the remaining 34. 2% is magnesium. Dalton proposed this: All matter is made up of individual particles called ‘atoms’, which cannot be divided. In other words he rediscovered Demokritos theory.

Dalton’s Theories of the Atom n All elements are composed of atoms. n All atoms of the same element have the same mass and atoms of different elements have different masses. n Compounds contain atoms of more than one element. n In a particular compound, atoms of different elements always combine in the same way.

Thomson’s Model of the Atom Joseph John Thomson or JJ Thomson. n Conducted experiments with a device called a Cathode Ray Tube or CRT. n Using it, he determined that neutrally or uncharged atoms actually contained charged part n So he discovered the atom WAS divisible. n

Plum pudding model n This was an old dessert popular in England at Thomson’s time. n n Raisins were stuck in bread pudding. The raisins were negative particles while the pudding was the positive portion. The correct balance between these two made atoms neutral. If developed today, he’d of used chocolate chip cookie dough.

Rutherford’s Atomic Theory n n An old student of JJ’s was Ernest Rutherford. Ernest devised an experiment in which he was going to prove his old teacher’s plum pudding model correct. This experiment was called the ‘Gold foil’ experiment. The result of this experiment was the positive ‘pudding’ portion of the atom was much, much smaller than thought. This very small, dense, positive center of the atom he called the ‘nucleus’ which means kernel in Latin. (Think of the kernel of popcorn. ) What this also means is that the atom is made mostly of empty space with over 99% of the mass located in the nucleus. If the nucleus of an atom were the size of a marble and placed at the 50 yard line at Michigan Stadium, the nearest electron would be at the top row of the stadium!

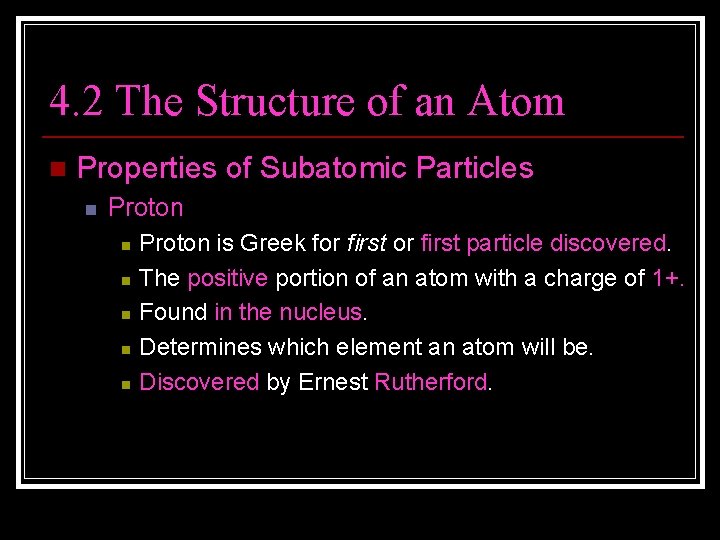
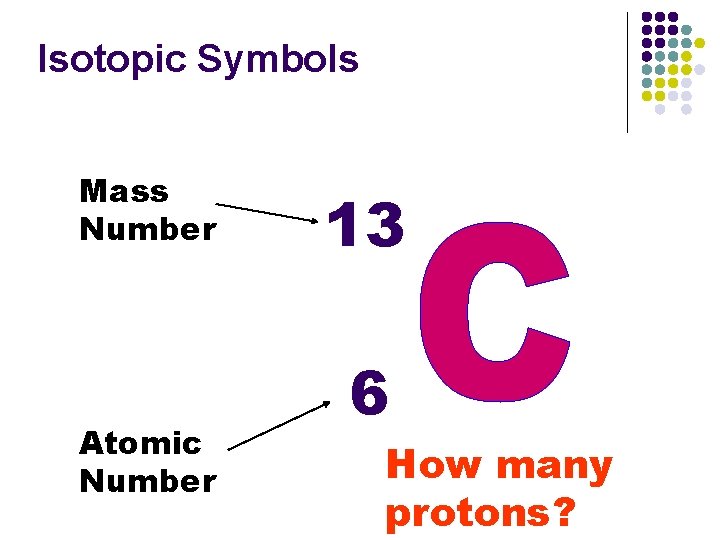
4. 2 The Structure of an Atom n Properties of Subatomic Particles n Proton n n Proton is Greek for first particle discovered. The positive portion of an atom with a charge of 1+. Found in the nucleus. Determines which element an atom will be. Discovered by Ernest Rutherford.

4. 2 (continued) n Neutron n n Particle found in the nucleus. Neutrally charged particle. ~same size as a proton. Discovered by James Chadwick.

4. 2 (continued) n Electrons n n The negative portion of an element with a charge of 1 -. Same magnitude as that of a proton. Much smaller than a proton/neutron…. ~1/2, 000. Despite its small size, an electron is as important as a proton. Named after Greek word meaning ‘amber’. The Greeks found that when amber was rubbed, it would obtain a charge.

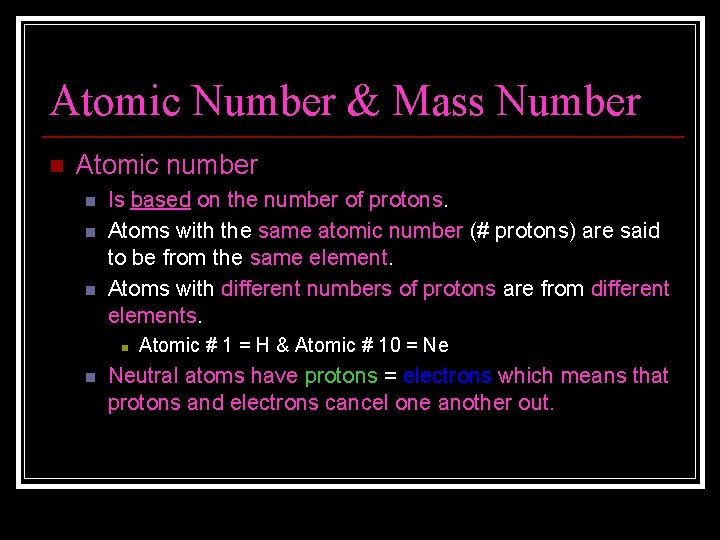
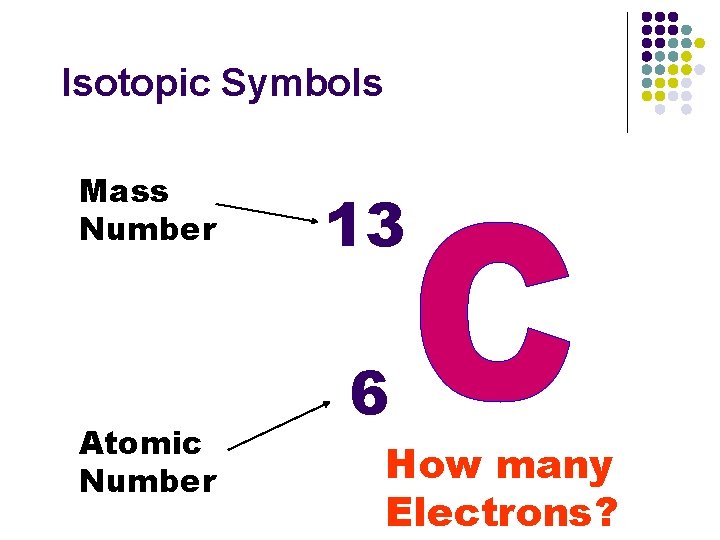
Atomic Number & Mass Number n Atomic number n n n Is based on the number of protons. Atoms with the same atomic number (# protons) are said to be from the same element. Atoms with different numbers of protons are from different elements. n n Atomic # 1 = H & Atomic # 10 = Ne Neutral atoms have protons = electrons which means that protons and electrons cancel one another out.

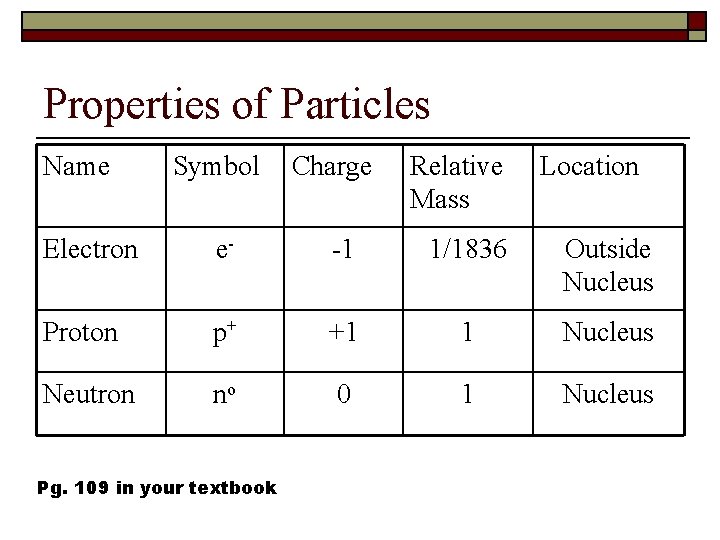
Properties of Particles Name Symbol Charge Relative Mass Location Electron e- -1 1/1836 Outside Nucleus Proton p+ +1 1 Nucleus Neutron no 0 1 Nucleus Pg. 109 in your textbook

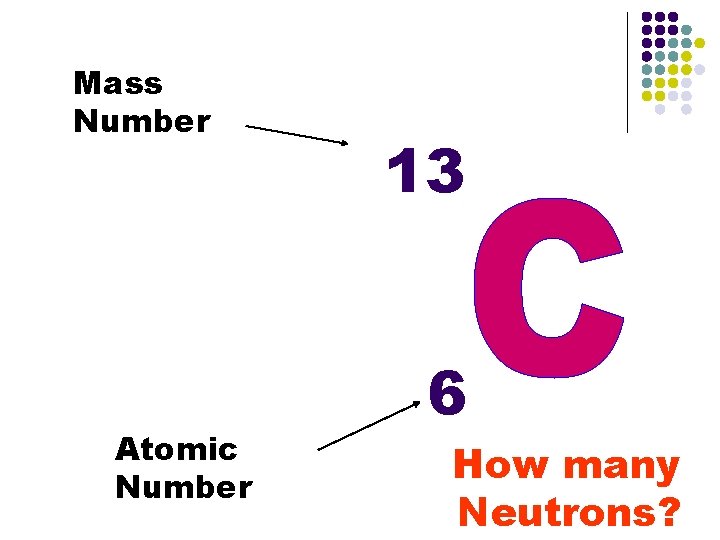
Mass number This is a number that represents how big the nucleus is. n So then Mass # = p+ + no n If Mass # = p+ + no and Atomic # = p+ then…. Mass # - Atomic # = no n

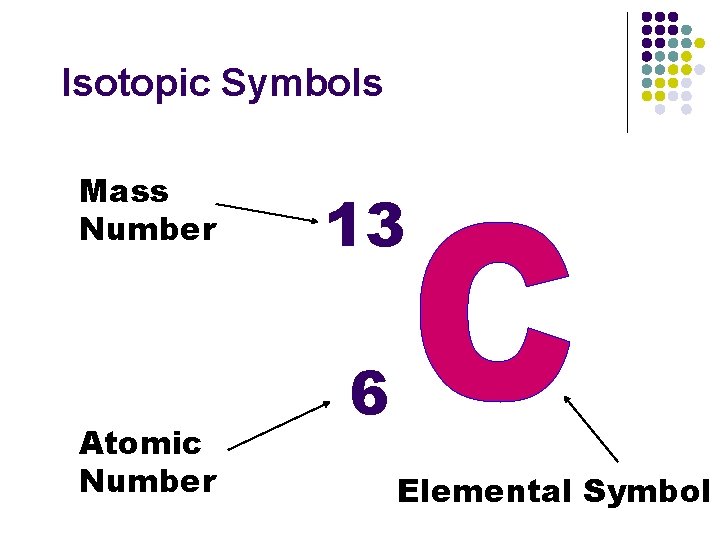
Isotopic Symbols Mass Number Atomic Number 13 6 Elemental Symbol

Isotopic Symbols Mass Number Atomic Number 13 6 How many protons?

Mass Number Atomic Number 13 6 How many Neutrons?

Isotopic Symbols Mass Number Atomic Number 13 6 How many Electrons?

Isotopes Atoms of the same element will ALWAYS have the same number of protons. n Sometimes the #’s of neutrons will be different…. these are called isotopes. n n Example: An atom of Carbon has mass # = 12. Another atom of C has a mass # = 14. They are each a carbon atom, but the isotope of carbon with a mass # of 14 has 2 extra neutrons.

Isotopes n n n Most elements have one or more isotopes. There is usually a low percentage of these isotopes…. like 1% or fewer of atoms have isotopes. The two isotopes of carbon can be represented in one of two ways: n n n C-12 & C-14 12 C & 14 C 12 C exists 98. 5% & 14 C exists 1. 5%.

4. 3 Modern Atomic Theory After Rutherford’s model came the Bohr model for the atom. l Rutherford talked about the nucleus, but Bohr decided to focus on electrons. l See page 114 to see all of the different models of the atom that have been known to this point. l

Bohr model l Energy levels • He thought electrons simply orbited around • • the nucleus like planets around the Sun. Planets are pulled around the Sun by the force of gravity. Electrons go around the nucleus because of the attraction between the positive nucleus and negative electrons.

Energy levels l l l Electrons have energy because they are in motion. The more NRG an electron has the farther it can be from the nucleus. The less energy, the closer it will be to the nucleus. We say electrons having more energy are at a higher energy level. Those electrons have less energy are at a lower energy level.

Energy levels are like steps on a staircase.

– Must stand on a step; you cannot be between steps! – Same for electrons and their energy levels. – The landing or bottom of the steps would be closest to the nucleus. – Electrons can move between energy levels just like you can go up & down the stairs to lunch.

§ Going up the stairs…. . more tiring than coming down?

Energy levels It is easier for an electron to go down from a higher NRG level to a lower…. lose NRG. More difficult for an electron to move up from a lower NRG level to a higher…. gain NRG. Just like you can jump up two or more steps at a time, electrons can jump up or fall down many energy levels…depends on how much energy is given to or given off by the electron.

Evidence for energy levels When electrons drop to a lower energy level, they again, release energy. This energy can be seen, sometimes, as light we can see with our eyes. This is why neon lights glow. When copper is placed in fire, the flames turn green.

Electron Cloud model n n n Bohr’s model was good, but again, someone was able to do it better. Electrons don’t go around the nucleus the way Bohr thought. It was found that electrons move around the nucleus at the speed of light. They whirl around in a pattern. The pattern formed is similar to what you would see if you looked at a fan spinning real fast.

Electron cloud model (continued) n These whirling electrons appear as a ‘blur’ or ‘cloud’. This is why we call this model of the atom the ‘cloud’ model.

Atomic orbitals n n n An orbital is a region in space in which an electron is most likely to be found. It is an approximation of where an electron is ‘most’ of the time. Do you spend all of your time in Big Rapids? Some in B. R. , just outside the city, out of the county, out of the state, etc. Where you are the most would be representative of your orbital.

Atomic orbitals Orbitals have shapes. n The farther you get from the nucleus, the more room there is for electrons. n The more room there is for electrons, the more orbitals there can be. n Each orbital can have only 2 electrons. n

Atomic orbitals Energy Levels 1 # Orbitals Max. # Electrons 1 2 2 4 8 3 9 18 4 16 32

Electron configurations n The arrangement of electrons in the orbitals of an atom is called an electron configuration.

Electron configurations (continued) n. A ‘configuration’ has to do with how something is arranged…like chairs in this room.

Electron configurations n The most stable electron configuration is the one in which the electrons are in orbitals with the lowest amount of energy.

Electron configurations (continued) n When all electrons are as low in NRG as possible, the atom is said to be in its ground state.

Electron configurations (continued) n When electrons ‘jump’ to a higher energy level, they are said to be in an excited state.

Electron configurations (continued) n Electrons will always fall back to a ground state eventually.

Electron configurations (contiuned) n When they do, NRG is released…. we see different colors of light. Green, blue, red, orange, any colors !!!! Watch the demonstration!!!