Chemistry I Honors Structure and Properties Intermolecular Forces





- Slides: 12

Chemistry I Honors Structure and Properties Intermolecular Forces

What are IMF’s • These are forces of attraction that exist between the representative particles of pure substances. • We typically recognize six different types of these forces. • These IMF’s give pure substances many of their physical properties. • We use a knowledge of the IMF’s to explain why different substances have differences in these characteristics.

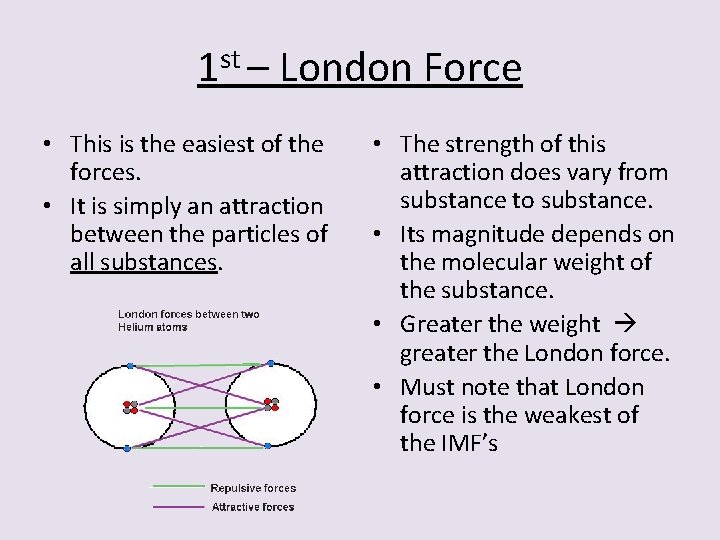
1 st – London Force • This is the easiest of the forces. • It is simply an attraction between the particles of all substances. • The strength of this attraction does vary from substance to substance. • Its magnitude depends on the molecular weight of the substance. • Greater the weight greater the London force. • Must note that London force is the weakest of the IMF’s

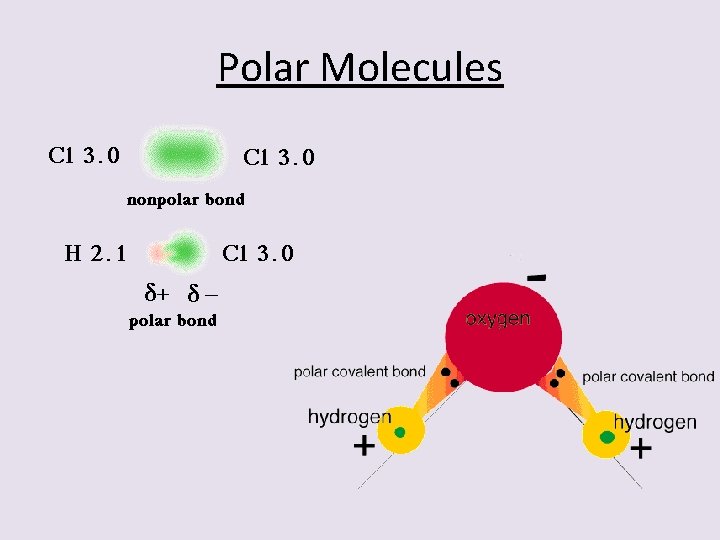
2 nd – Dipole-Dipole Interaction • Strange name !! • First – all polar molecules are said to possess a “dipole”. • This means that they have two poles (ends that are relatively positive and negatively charged. • We have already seen how a molecule can be polar. – Difference in electronegativity between the atoms that are bonded. – Presence of one or more unbalanced lone pairs of electrons on the central atom.

Polar Molecules

What Happens? • In dipole-dipole interaction, the oppositely charged ends of polar molecules are attracted to each other. • This IMF is stronger than the London force described earlier. • Also, it only applies to polar covalent substances.

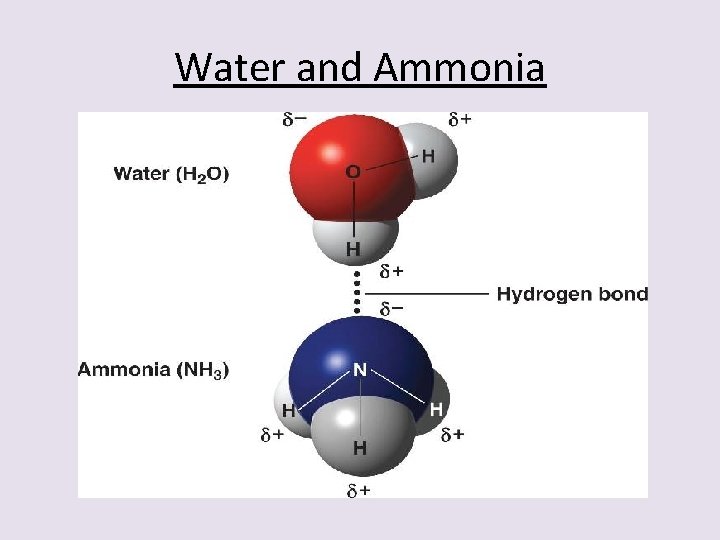
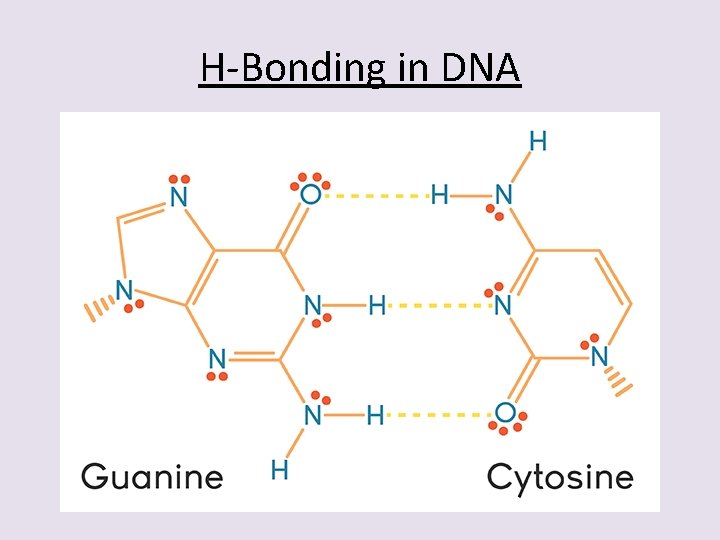
3 rd – Hydrogen Bonding • This is a very specialized form of dipole-dipole interaction. • The molecules involved have very specific requirements of their composition. • Hydrogen bonding is stronger than dipole interaction because it is more of a chemical bond than just an attraction force. • The hydrogen bond is a half-strength covalent bond that forms between a Hydrogen atom on one polar molecule and the F, O, or N atom of a 2 nd polar molecule.

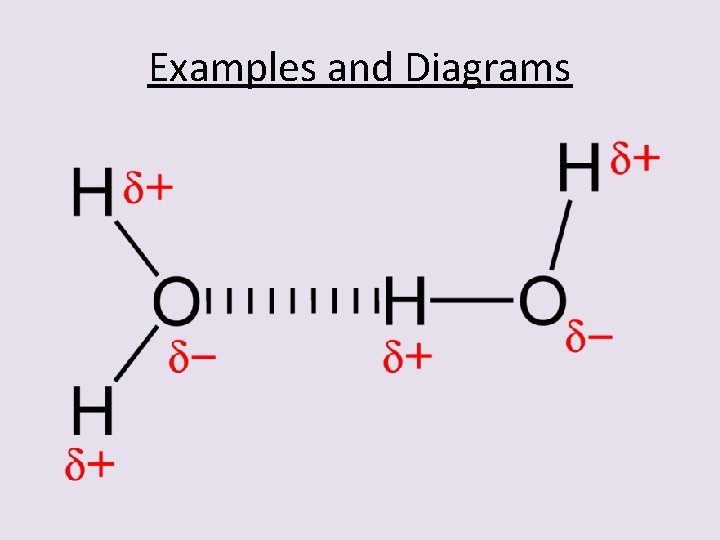
Examples and Diagrams

Water and Ammonia

H-Bonding in DNA

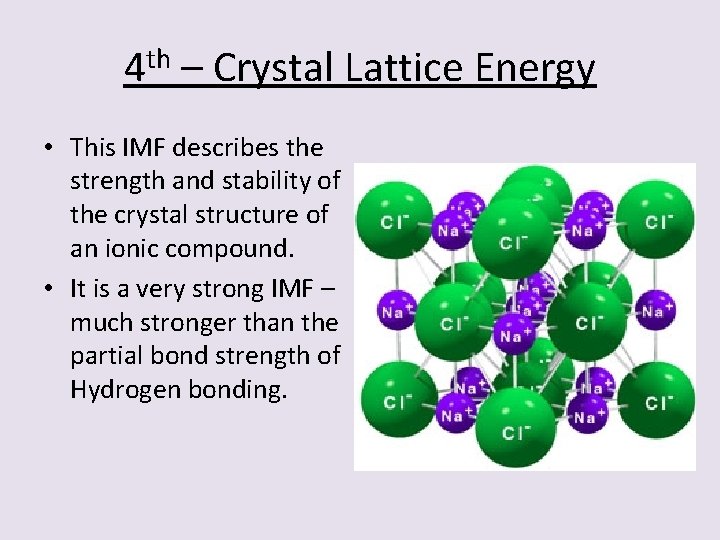
4 th – Crystal Lattice Energy • This IMF describes the strength and stability of the crystal structure of an ionic compound. • It is a very strong IMF – much stronger than the partial bond strength of Hydrogen bonding.

Last Two IMF’s • IMF #5 is Metallic Bonding • IMF #6 is Covalent – the same type of Networking. bonding that was • Also described in previous described in the last lesson on Classification of • Generally regarded as the Substances. strongest of the IMF’s. • There is variation in the strength of this IMF, but it is commonly regarded as being as strong as Crystal Lattice Energy.