Chemistry Grade 910 Review Outline What is Chemistry










- Slides: 18

Chemistry Grade 9/10 Review

Outline What is Chemistry? So What? Classification of Matter Properties of Matter Elements Periods Chemical Families Metals & Non-Metals Subatomic Particles

So What? Chemistry is part of our everyday lives. The products of chemical processes and reactions are involved in all aspects of our lives: – The clothes we wear – The food we eat – The places we live and work

Classifying Matter is classified as either pure substances or mixtures. Matter Pure Substances Elements Compounds Mixtures Solutions Heterogeneous Mixtures

Pure Substances - contain only one kind of particle. Elements - are pure substances that cannot be broken down into simpler substances. (eg. gold) Compounds - contain two or more elements in fixed proportions. (eg. water – H 20, carbon dioxide – C 02)

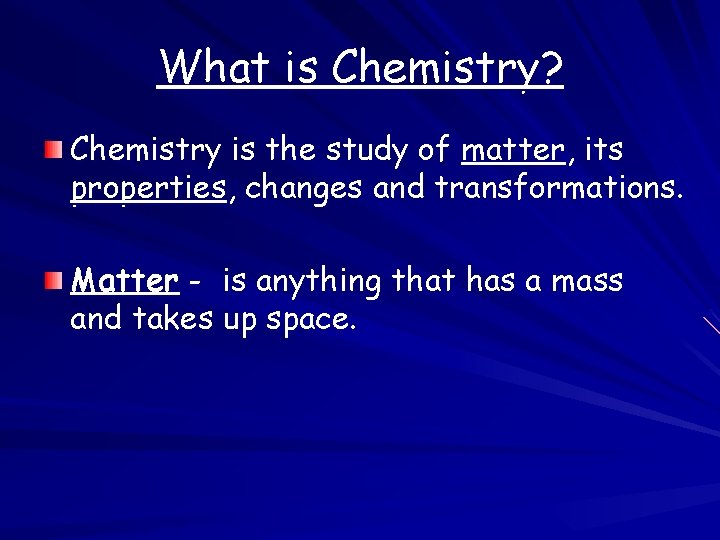
What is Chemistry? Chemistry is the study of matter, its properties, changes and transformations. Matter - is anything that has a mass and takes up space.

Mixtures - contain two or more pure substances. Solutions - have only one visible component. (eg. apple juice) Heterogeneous mixtures contain two or more visible components. (eg. balsamic salad dressing)

Properties of Matter Physical Properties - are characteristics of a substance. Some physical properties of matter are: – State (solid, liquid, gas) – Colour - Odour - Texture - Mass – Volume - Melting Point – Boiling Point

Properties of Matter Chemical Properties - are characteristic behaviours of a substance. Some chemical properties of matter are: – Combustibility – Reaction with acids – Flammability

Elements are pure substances that cannot be broken down into a simpler substance. – Eg. gold, nickel, hydrogen Elements are grouped on the Periodic Table according to physical and chemical properties.

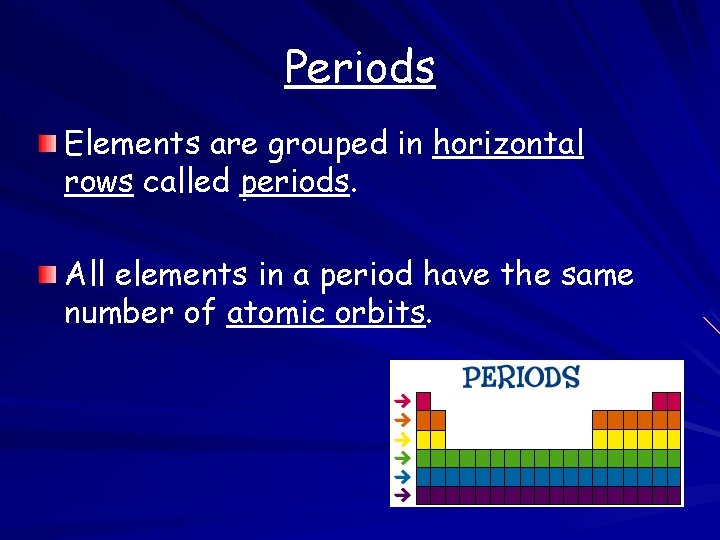
Periods Elements are grouped in horizontal rows called periods. All elements in a period have the same number of atomic orbits.

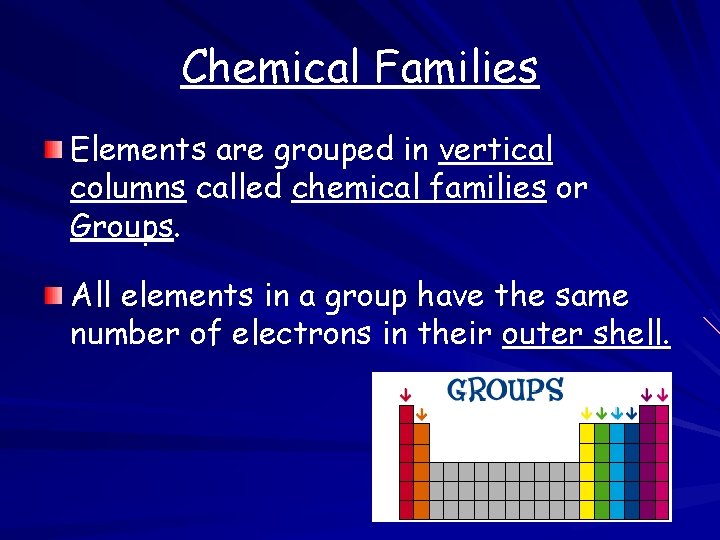
Chemical Families Elements are grouped in vertical columns called chemical families or Groups. All elements in a group have the same number of electrons in their outer shell.

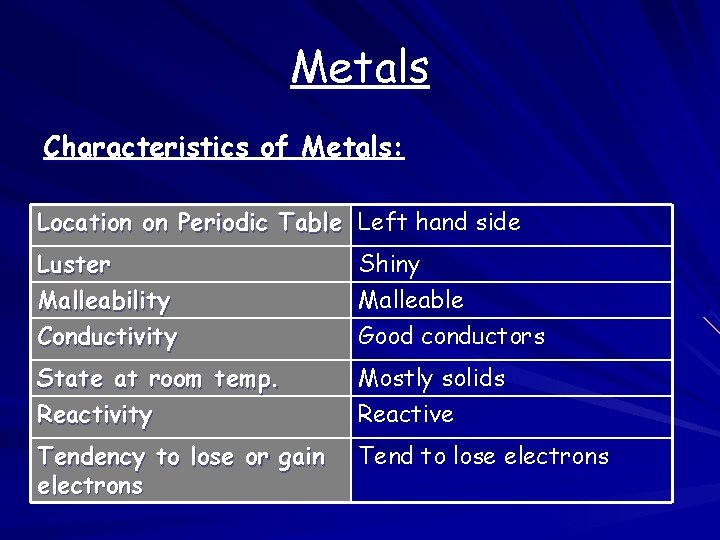
Metals Characteristics of Metals: Location on Periodic Table Left hand side Luster Malleability Conductivity Shiny Malleable Good conductors State at room temp. Reactivity Mostly solids Reactive Tendency to lose or gain electrons Tend to lose electrons

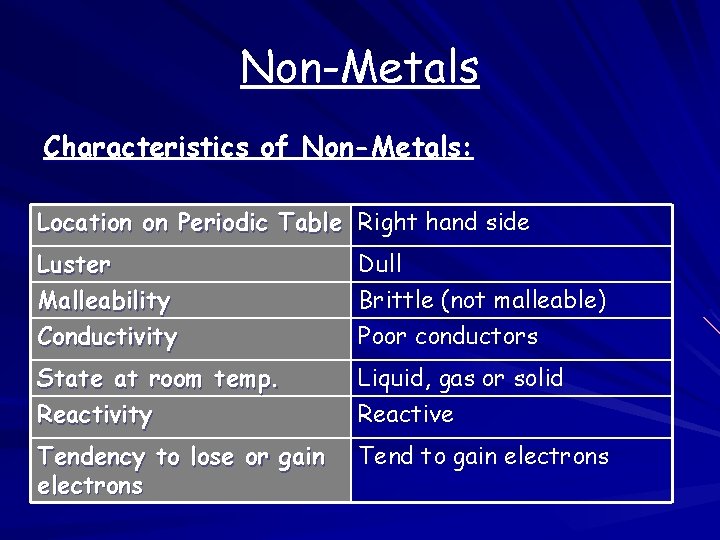
Non-Metals Characteristics of Non-Metals: Location on Periodic Table Right hand side Luster Malleability Conductivity Dull Brittle (not malleable) Poor conductors State at room temp. Reactivity Liquid, gas or solid Reactive Tendency to lose or gain electrons Tend to gain electrons

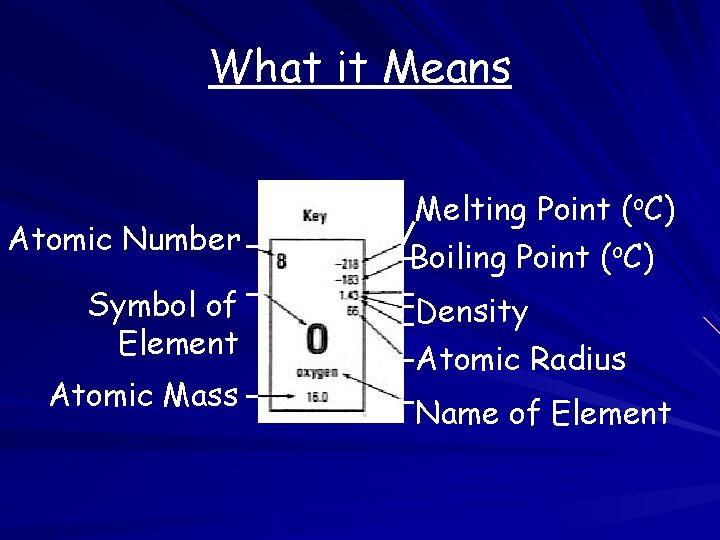
What it Means Atomic Number Symbol of Element Atomic Mass Melting Point (o. C) Boiling Point (o. C) Density Atomic Radius Name of Element

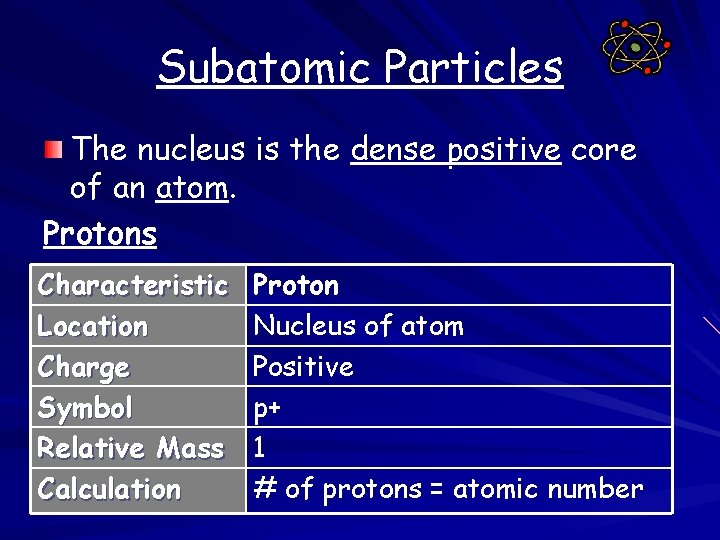
Subatomic Particles The nucleus is the dense positive core of an atom. Protons Characteristic Location Charge Symbol Relative Mass Calculation Proton Nucleus of atom Positive p+ 1 # of protons = atomic number

Subatomic Particles Neutron Characteristic Location Charge Symbol Relative Mass Calculation Neutron Nucleus of atom Neutral n 1 # of neutrons = atomic mass - # of protons

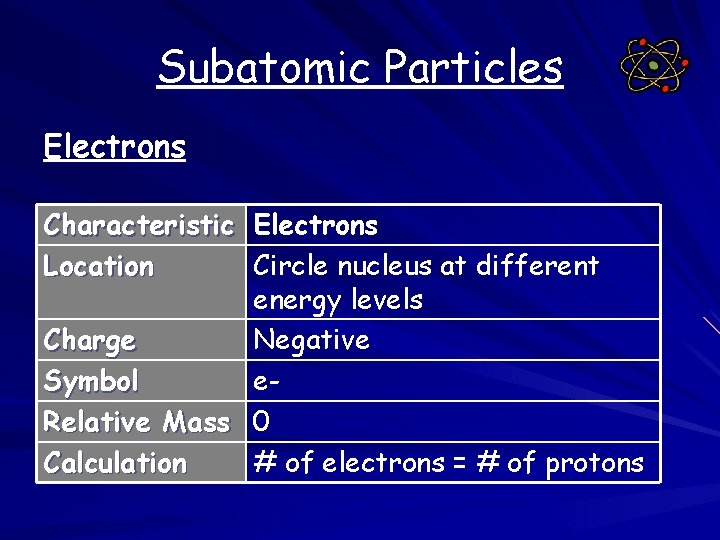
Subatomic Particles Electrons Characteristic Electrons Location Circle nucleus at different energy levels Charge Negative Symbol e. Relative Mass 0 Calculation # of electrons = # of protons