Chemistry Do Now 12 4 17 Directions Use














- Slides: 20

Chemistry Do Now 12 -4 -17 • Directions: Use your notes to calculate the molar mass of each compound. • • Au 2(SO 4)3 • Fe(CN)3 • C 8 H 18

Chemistry Do Now 12 -4 -17 Key • Directions: Use your notes to calculate the molar mass of each compound. • • Au 2(SO 4)3 – 2(197)+3(32)+12(16) = 682 g/mol • Fe(CN)3 – 1(56)+3(12)+3(14) = 134 g/mol • C 8 H 18 – 8(12)+18(1) = 114 g/mol

Objective • Students will know calculate the calculate change in heat problems and thermochemical problems by taking notes, guided practice and independent practice problem solving. • Mastery Level: 70% or better

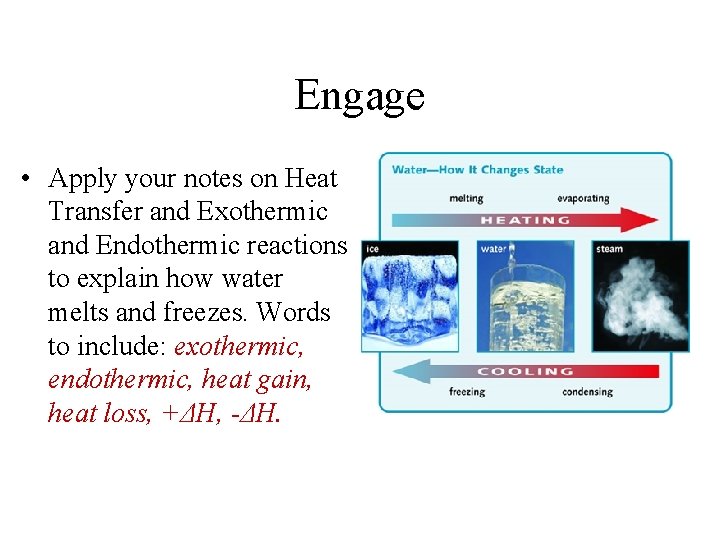
Engage • Apply your notes on Heat Transfer and Exothermic and Endothermic reactions to explain how water melts and freezes. Words to include: exothermic, endothermic, heat gain, heat loss, +ΔH, -ΔH.

Engage – Sample Response • Melting ice is an endothermic process because the ice is gaining heat as it melts. Therefore, the process has a positive delta H. The freezing of water into ice is an exothermic process because the liquid water loses heat as it freezes. Therefore, the process has a negative delta H.


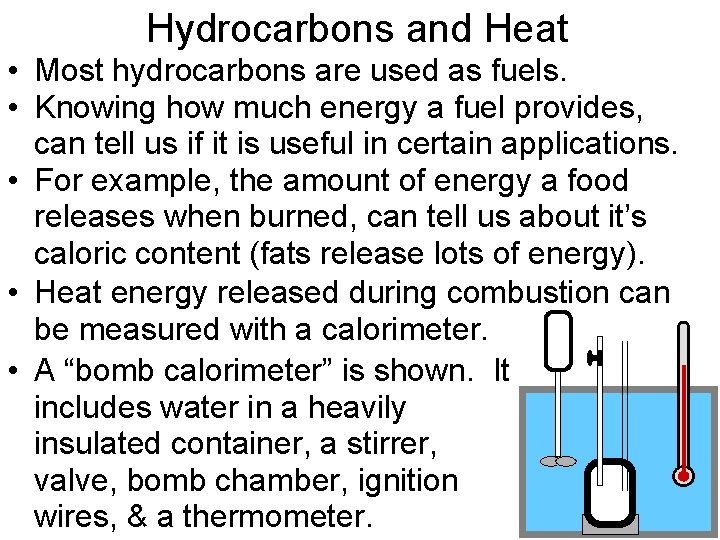
Hydrocarbons and Heat • Most hydrocarbons are used as fuels. • Knowing how much energy a fuel provides, can tell us if it is useful in certain applications. • For example, the amount of energy a food releases when burned, can tell us about it’s caloric content (fats release lots of energy). • Heat energy released during combustion can be measured with a calorimeter. • A “bomb calorimeter” is shown. It includes water in a heavily insulated container, a stirrer, valve, bomb chamber, ignition wires, & a thermometer.

Exothermic and Endothermic changes • The change in heat of the water tells us about the reaction of the chemicals. • An increase in water temperature indicates that the chemicals released energy when they reacted. This is called an “exothermic” reaction. • In an “endothermic” reaction, water temperature decreases as the chemicals absorb energy. • Heat is measured in Joules (J) or kilo. Joules (k. J). Before we do any heat calculations, you should know several terms …

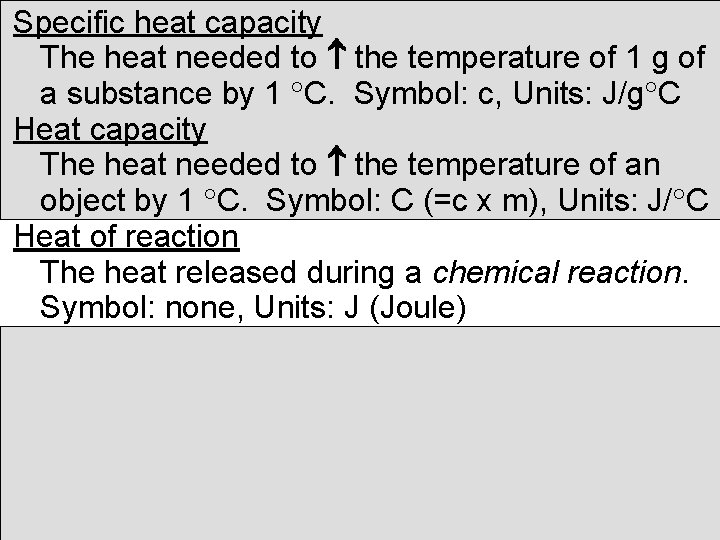
Specific heat capacity The heat needed to the temperature of 1 g of a substance by 1 C. Symbol: c, Units: J/g C Heat capacity The heat needed to the temperature of an object by 1 C. Symbol: C (=c x m), Units: J/ C Heat of reaction The heat released during a chemical reaction. Symbol: none, Units: J (Joule)

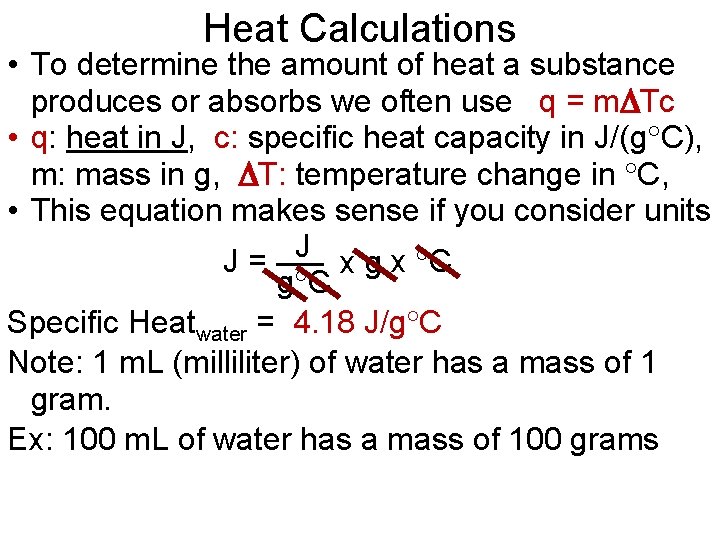
Heat Calculations • To determine the amount of heat a substance produces or absorbs we often use q = m Tc • q: heat in J, c: specific heat capacity in J/(g C), m: mass in g, T: temperature change in C, • This equation makes sense if you consider units J J= x g x C g C Specific Heatwater = 4. 18 J/g C Note: 1 m. L (milliliter) of water has a mass of 1 gram. Ex: 100 m. L of water has a mass of 100 grams

What does each variable mean in the heat formula, q = m ΔTc? • q = heat lost or gained • ΔT = change in temperature (Tfinal- Tinitial) • m = mass of object heated/cooled • c = specific heat capacity You will be given all but one of the above variables and asked to find the missing variable.

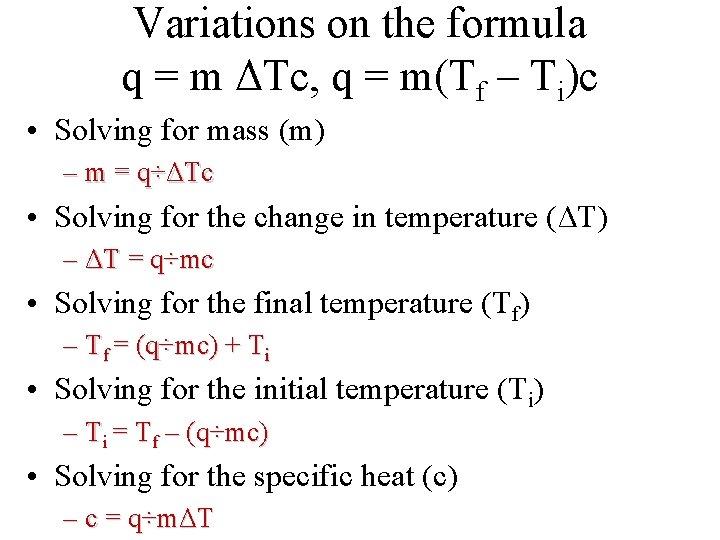
Variations on the formula q = m ΔTc, q = m(Tf – Ti)c • Solving for mass (m) – m = q÷ΔTc • Solving for the change in temperature (ΔT) – ΔT = q÷mc • Solving for the final temperature (Tf) – Tf = (q÷mc) + Ti • Solving for the initial temperature (Ti) – Ti = Tf – (q÷mc) • Solving for the specific heat (c) – c = q÷mΔT

Guided Practice Problem #1 • A student must use 225 m. L of hot water in a lab procedure. Calculate the amount of heat required to raise the temperature of 225 m. L of water from 20. 0 o. C to 100. 0 o. C.

Guided Practice Problem #2 • Calculate the specific heat capacity of a new alloy if a 15. 4 g sample absorbs 393 J when it is heated from 0. 0 o. C to 37. 6 o. C.

Guided Practice Problem #3 • A 40. 0 g sample of ethanol releases 2952 J as it cools from 50. 0 °C. Calculate the final temperature of the ethanol. • Specific Heat of Ethanol: 2. 460 J/g C

Guided Practice Problem #4 • Calculate the heat change associated with cooling a 350. 0 g aluminum bar from 70. 0 o. C to 25. 0 o. C. Is the change endothermic or exothermic? Why? • Specific heat of Aluminum: 0. 900 J/g C

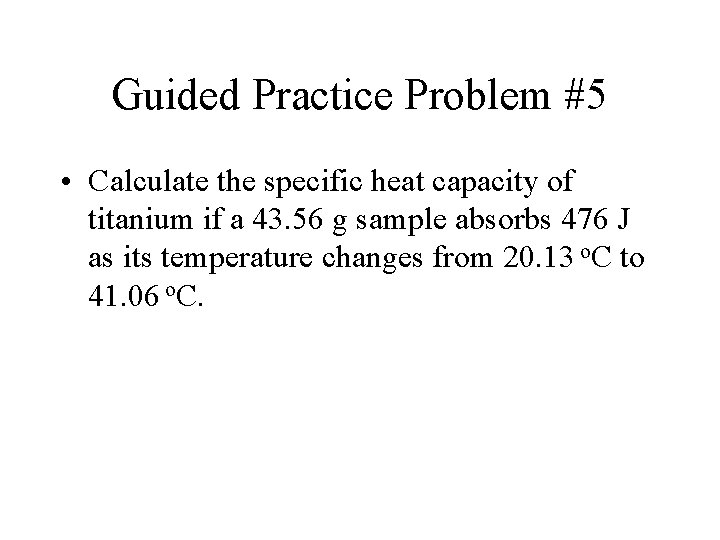
Guided Practice Problem #5 • Calculate the specific heat capacity of titanium if a 43. 56 g sample absorbs 476 J as its temperature changes from 20. 13 o. C to 41. 06 o. C.

Guided Practice Problem #6 • A 63. 5 g sample of an unidentified metal absorbs 355 J of heat when its temperature changes by 4. 56 o. C. Calculate the specific heat capacity of the metal.

Guided Practice Problem #7 • 750. 0 g of water that was just boiled (heated to 100. 0 o. C) loses 78, 450 J of heat as it cools. What is the final temperature of the water?

Guided Practice Problem #8 • A 175 g piece of iron and a 175 g piece of aluminum are placed in a hot water bath so that they are warmed to 99. 7 o. C. The metal samples are removed and cooled to 21. 5 o. C. Which sample undergoes the greater heat change? • Specific Heat Values: – Fe: 0. 444 J/g C – Al: 0. 900 J/g C