CHEMISTRY DMCU 1233 Fakulti Kejuruteraan Mekanikal UTe M









![Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Ca Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Ca](https://slidetodoc.com/presentation_image/32f5e0a9cab11e112a1d71dd6eff6812/image-31.jpg)
![Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or](https://slidetodoc.com/presentation_image/32f5e0a9cab11e112a1d71dd6eff6812/image-34.jpg)
- Slides: 35

CHEMISTRY - DMCU 1233 Fakulti Kejuruteraan Mekanikal, UTe. M Lecturer: IMRAN SYAKIR BIN MOHAMAD MOHD HAIZAL BIN MOHD HUSIN NONA MERRY MERPATI MITAN Electronic Structure of Atoms & Periodic Table Chapter 4 1

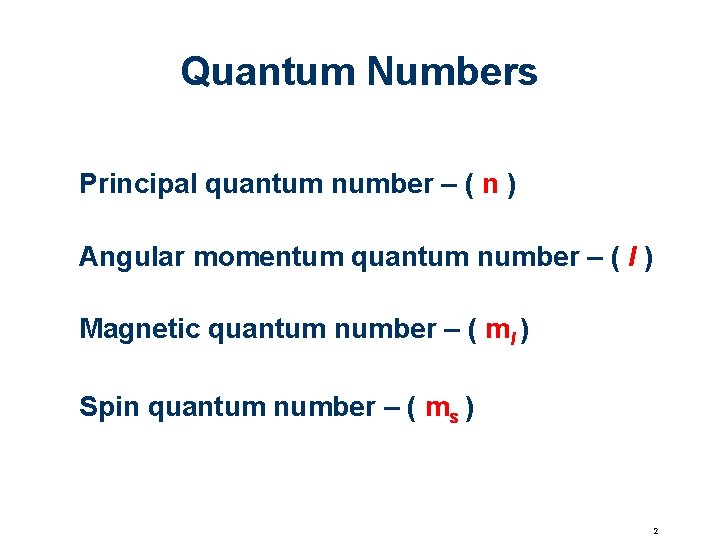
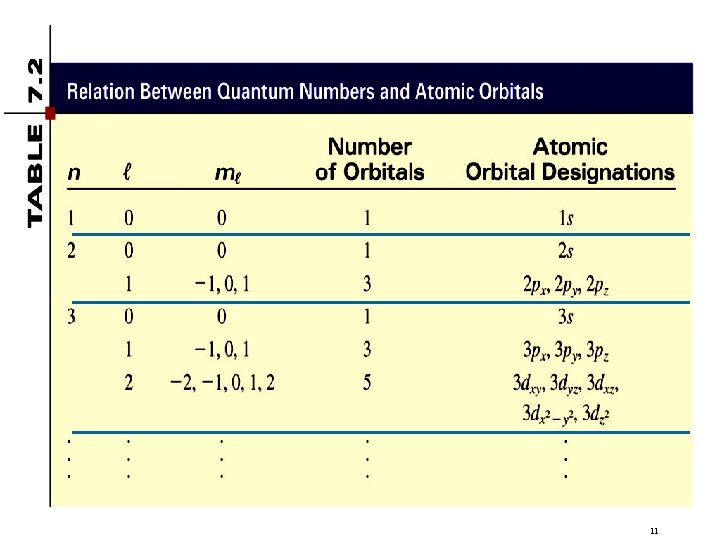
Quantum Numbers Principal quantum number – ( n ) Angular momentum quantum number – ( l ) Magnetic quantum number – ( ml ) Spin quantum number – ( ms ) 2

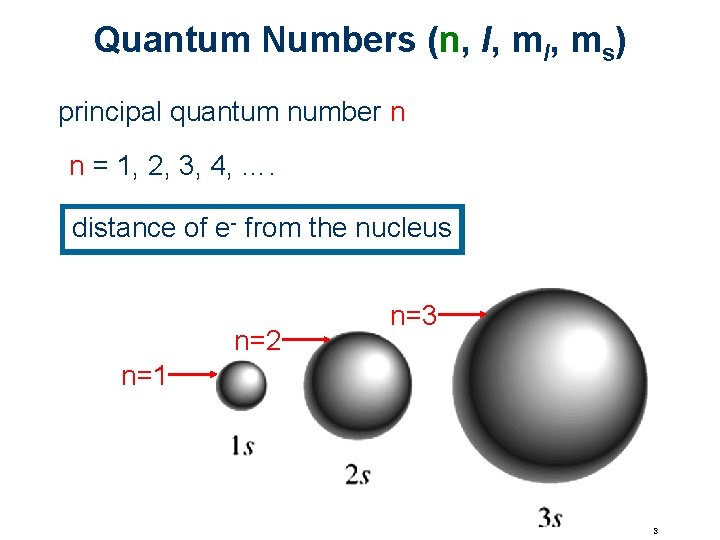
Quantum Numbers (n, l, ms) principal quantum number n n = 1, 2, 3, 4, …. distance of e- from the nucleus n=2 n=3 n=1 3

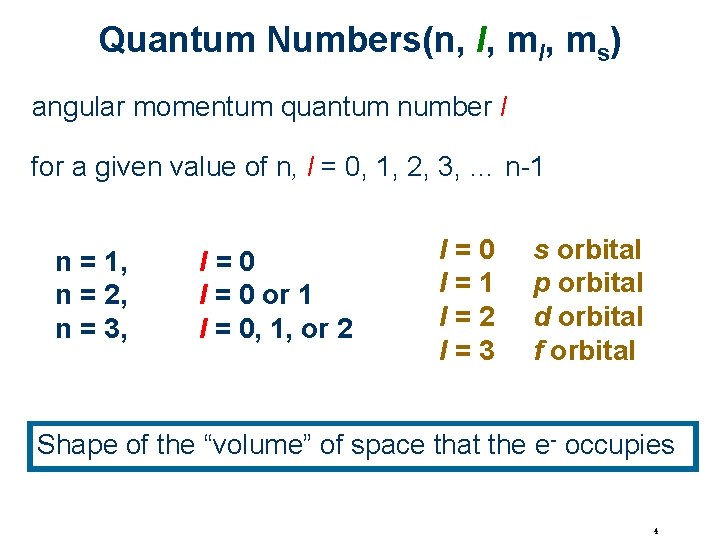
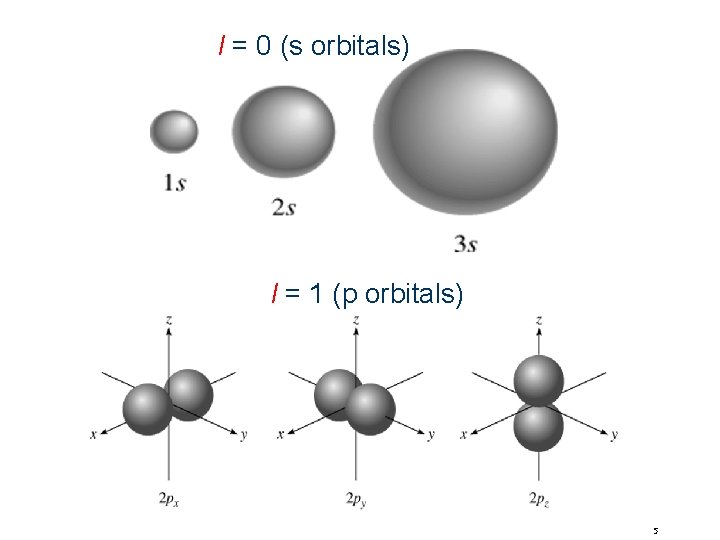
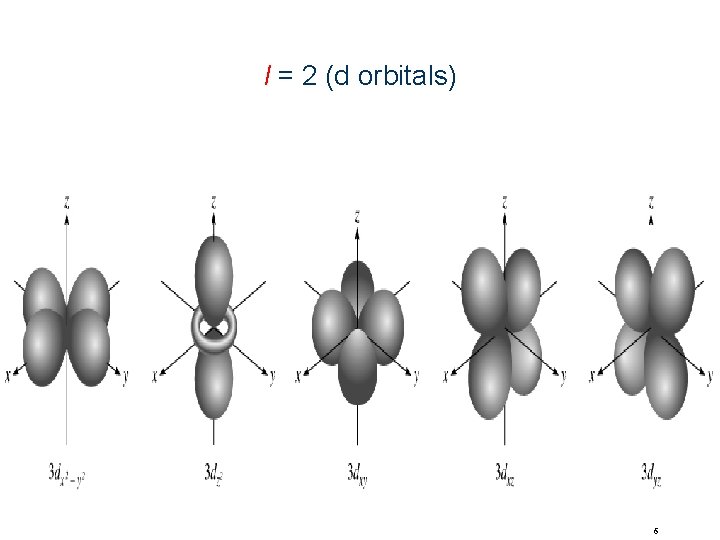
Quantum Numbers(n, l, ms) angular momentum quantum number l for a given value of n, l = 0, 1, 2, 3, … n-1 n = 1, n = 2, n = 3, l=0 l = 0 or 1 l = 0, 1, or 2 l=0 l=1 l=2 l=3 s orbital p orbital d orbital f orbital Shape of the “volume” of space that the e- occupies 4

l = 0 (s orbitals) l = 1 (p orbitals) 5

l = 2 (d orbitals) 6

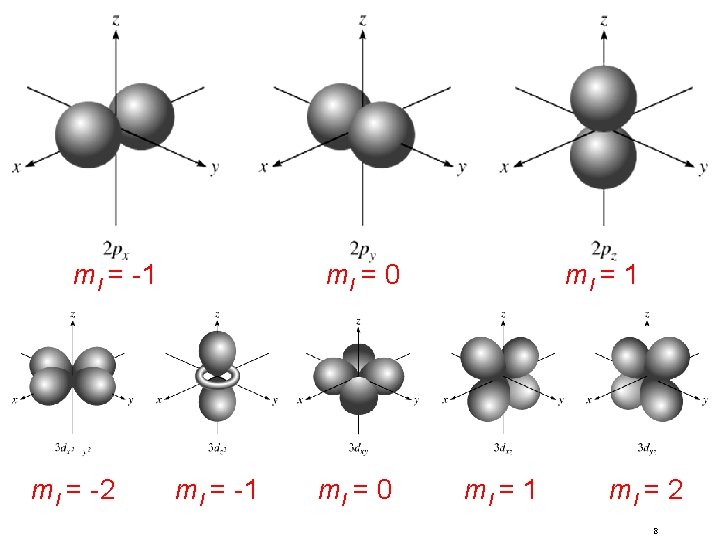
Quantum Numbers (n, l, ms) magnetic quantum number ml for a given value of l ml = -l, …. , 0, …. +l for l = 0 (s orbital) ml = 0 if l = 1 (p orbital), ml = -1, 0, or +1 if l = 2 (d orbital), ml = -2, -1, 0, +1, or +2 orientation of the orbital in space 7

ml = -1 ml = -2 ml = 0 ml = -1 ml = 0 ml = 1 ml = 2 8

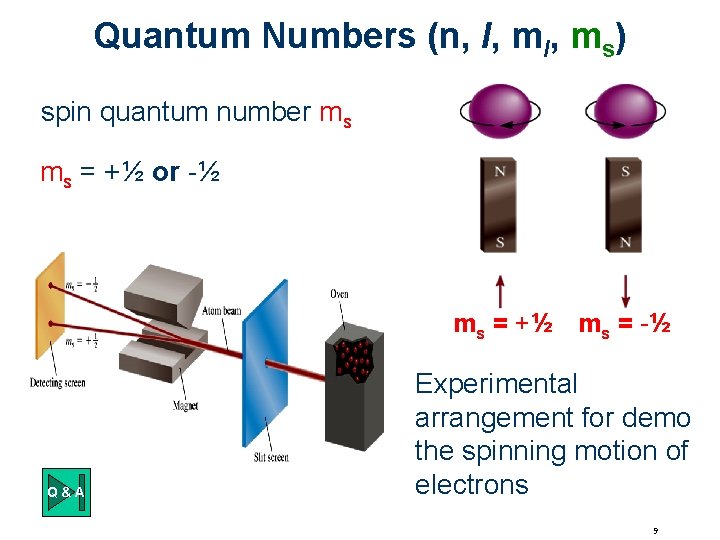
Quantum Numbers (n, l, ms) spin quantum number ms ms = +½ or -½ ms = +½ ms = -½ Q&A Experimental arrangement for demo the spinning motion of electrons 9

Quantum Numbers (n, l, ms) Existence (and energy) of electron in atom is described by its unique Quantum Numbers Pauli exclusion principle No two electrons in an atom can have the same four quantum numbers. 10

11

Quantum Numbers (n, l, ms) Shell – electrons with the same value of n Subshell – electrons with the same values of n and l Orbital – electrons with the same values of n, l, and ml How many electrons can an orbital hold? 12

How many 2 p orbitals are there in an atom? How many electrons can be placed in the 3 d subshell? Q&A 13

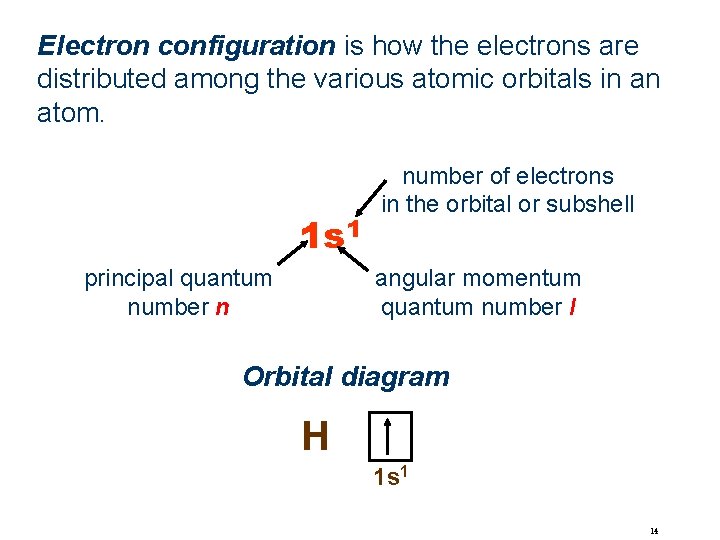
Electron configuration is how the electrons are distributed among the various atomic orbitals in an atom. 1 s 1 number of electrons in the orbital or subshell angular momentum quantum number l principal quantum number n Orbital diagram H 1 s 1 14

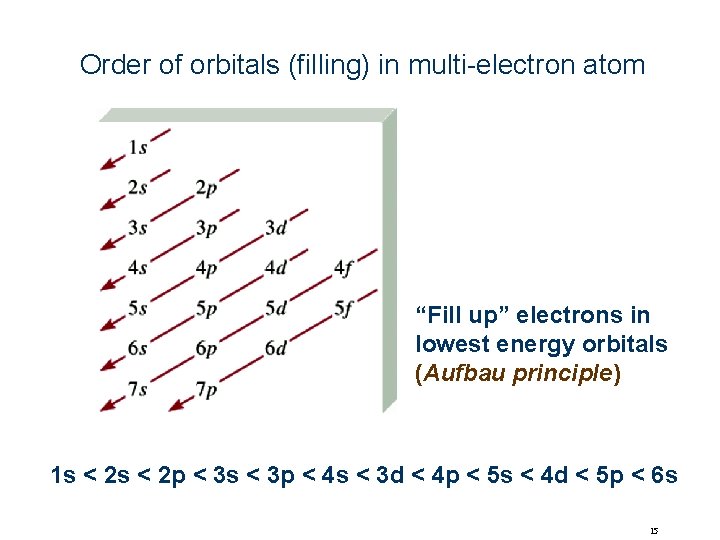
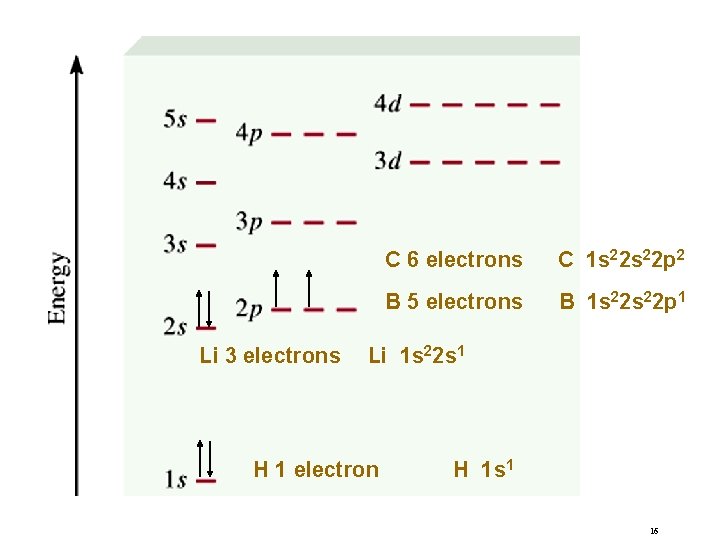
Order of orbitals (filling) in multi-electron atom “Fill up” electrons in lowest energy orbitals (Aufbau principle) 1 s < 2 p < 3 s < 3 p < 4 s < 3 d < 4 p < 5 s < 4 d < 5 p < 6 s 15

Li 3 electrons C 6 electrons C 1 s 22 p 2 B 5 electrons B 1 s 22 p 1 Li 1 s 22 s 1 H 1 electron H 1 s 1 16

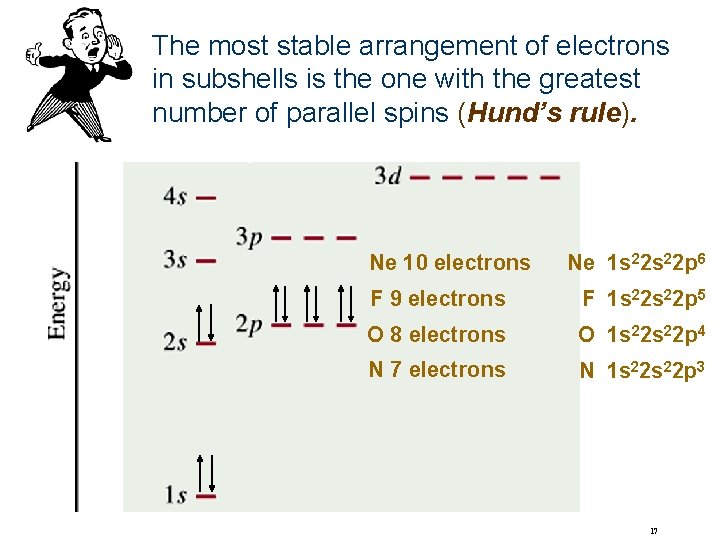
The most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins (Hund’s rule). Ne 10 electrons Ne 1 s 22 p 6 F 9 electrons F 1 s 22 p 5 O 8 electrons O 1 s 22 p 4 N 7 electrons N 1 s 22 p 3 17

What is the electron configuration of Mg? What are the possible quantum numbers for the last (outermost) electron in Cl? 18

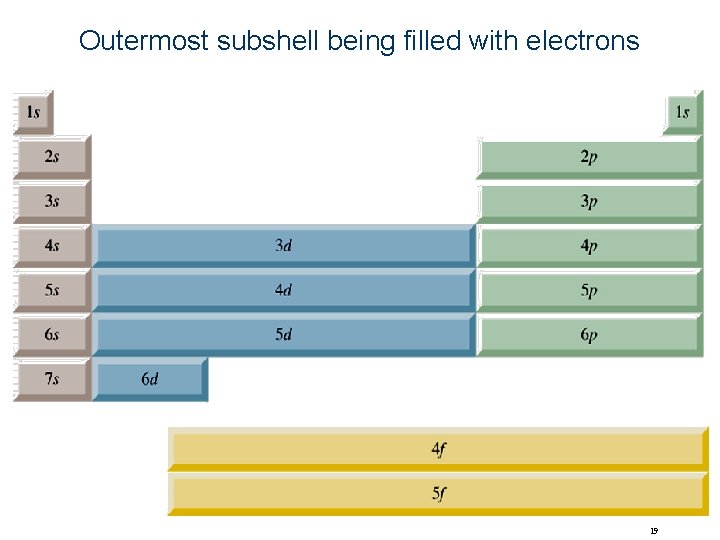
Outermost subshell being filled with electrons 19

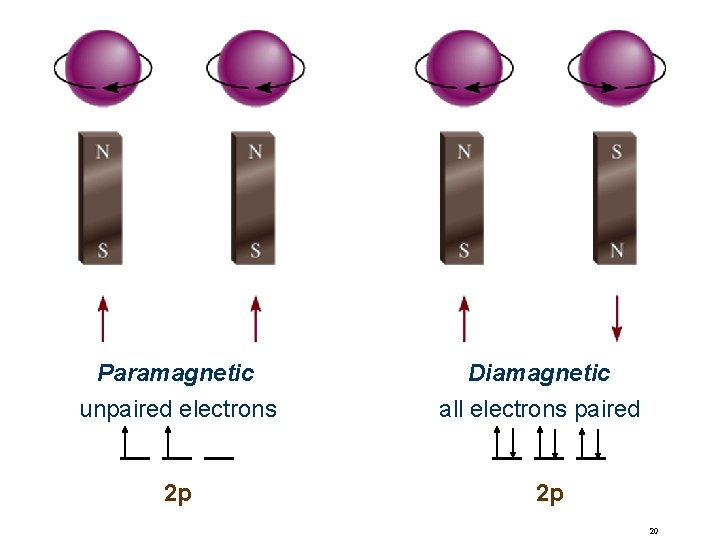
Paramagnetic unpaired electrons 2 p Diamagnetic all electrons paired 2 p 20

Q & A session Name the orbital described by the following quantum numbers : a. b. c. d. n n = = 3, 3, 3, 5, l l = = 0 1 2 0 21

Q & A session Give the n and l values for the following orbital a. 1 s b. 3 s c. 2 p d. 4 d e. 5 f What and the possible ml values for the following types of orbital? a. s b. p c. d d. f 22

Q & A session How many possible orbital are there for n = a. 4 b. 10 How many electrons can inhabit all of the n = 4 orbital? Place the following orbital in order of increasing energy: 1 s, 3 s, 4 s, 6 s, 3 d, 4 f, 3 p, 7 s, 5 d, 5 p 23

Write electron configurations for the following atoms: a. H Q & A session b. Li+ c. N d. F e. Ca 24

Q & A session Draw an orbital diagrams for atoms with the following electron configurations: 1 s 22 p 63 s 23 p 3 25

Periodic Table 26

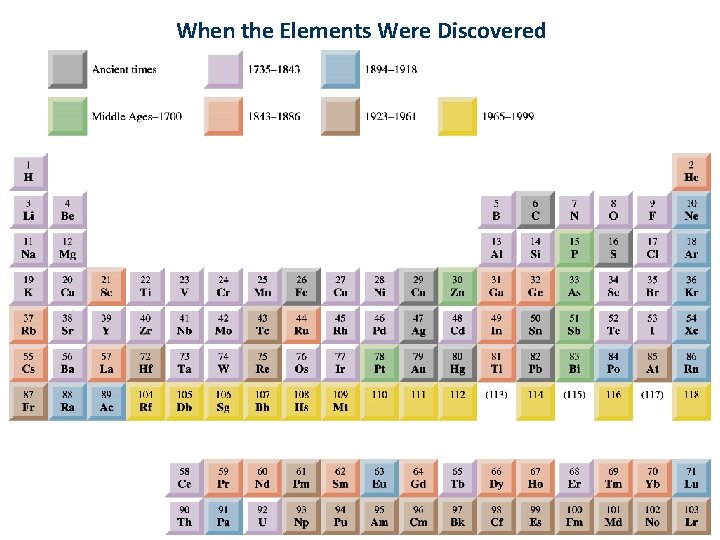
When the Elements Were Discovered 27

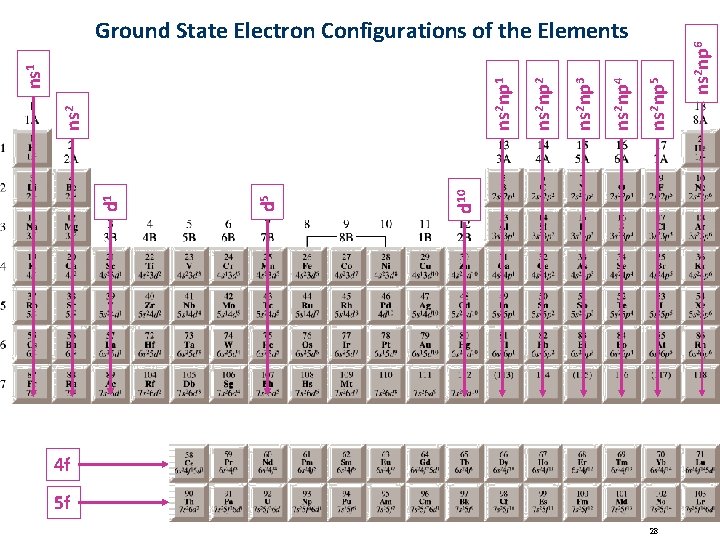
4 f 5 f 28 ns 2 np 6 ns 2 np 5 ns 2 np 4 ns 2 np 3 ns 2 np 2 d 10 d 5 d 1 ns 2 np 1 ns 1 Ground State Electron Configurations of the Elements

29

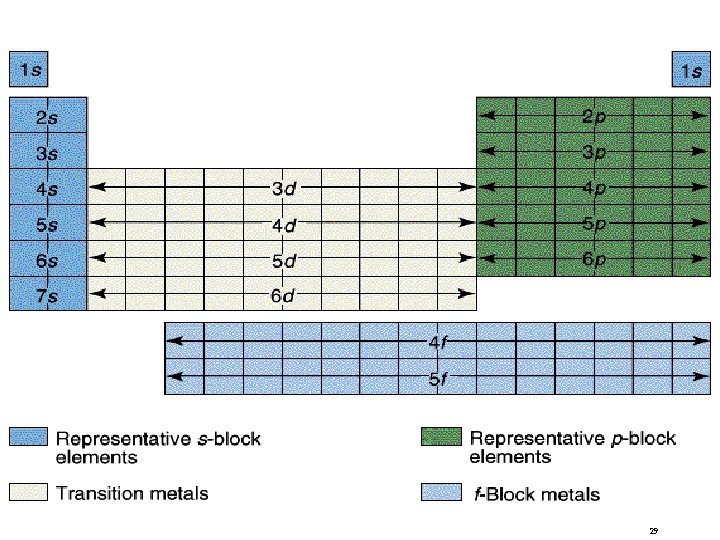
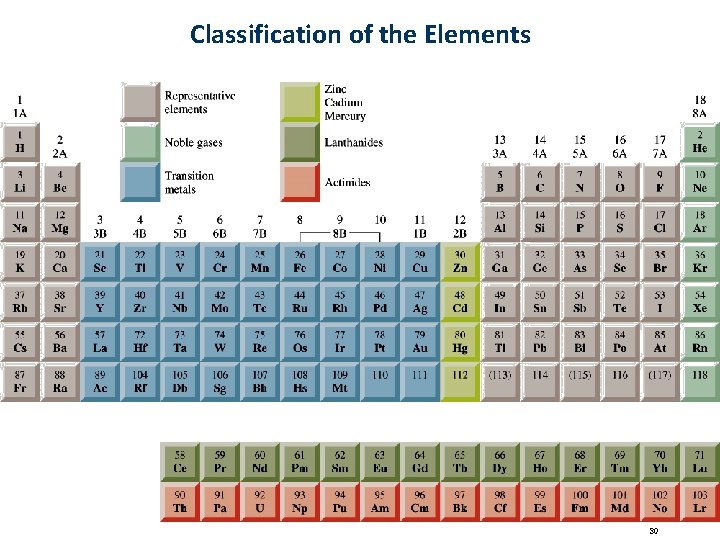
Classification of the Elements 30
![Electron Configurations of Cations and Anions Of Representative Elements Na Ne3 s 1 Ca Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Ca](https://slidetodoc.com/presentation_image/32f5e0a9cab11e112a1d71dd6eff6812/image-31.jpg)
Electron Configurations of Cations and Anions Of Representative Elements Na [Ne]3 s 1 Ca [Ar]4 s 2 Al [Ne]3 s 23 p 1 Na+ [Ne] Ca 2+ [Ar] Al 3+ [Ne] Atoms lose electrons so that cation has a noble-gas outer electron configuration. H 1 s 1 Atoms gain electrons so F 1 s 22 p 5 that anion has a noble-gas outer electron O 1 s 22 p 4 configuration. N 1 s 22 p 3 H- 1 s 2 or [He] F- 1 s 22 p 6 or [Ne] O 2 - 1 s 22 p 6 or [Ne] N 3 - 1 s 22 p 6 or [Ne] 31

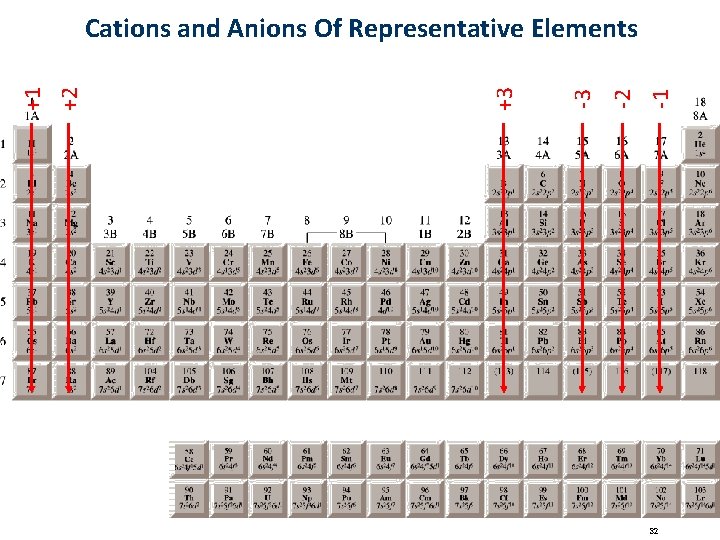
-1 -2 -3 +3 +2 +1 Cations and Anions Of Representative Elements 32

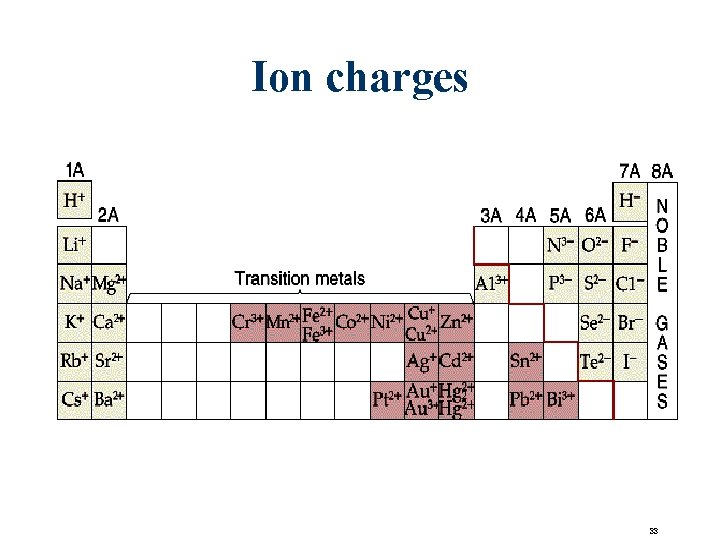
Ion charges 33
![Na Ne Al 3 Ne O 2 1 s 22 p 6 or Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or](https://slidetodoc.com/presentation_image/32f5e0a9cab11e112a1d71dd6eff6812/image-34.jpg)
Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or [Ne] F-: 1 s 22 p 6 or [Ne] N 3 -: 1 s 22 p 6 or [Ne] Na+, Al 3+, F-, O 2 -, and N 3 - are all isoelectronic with Ne What neutral atom is isoelectronic with H- ? 34

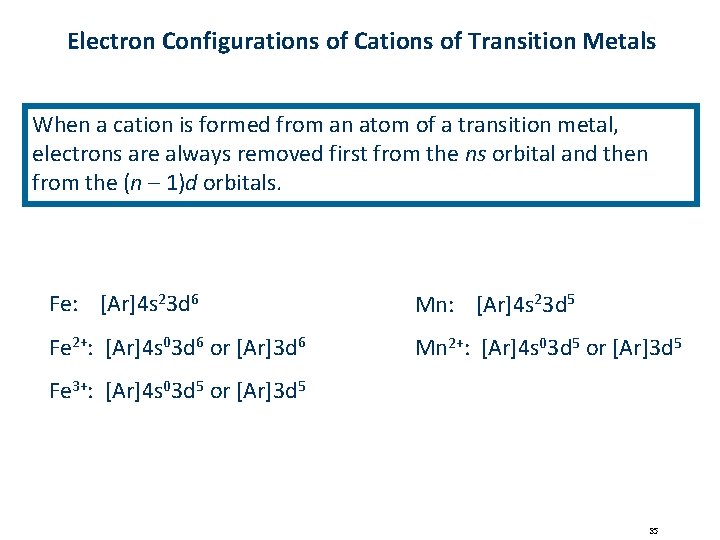
Electron Configurations of Cations of Transition Metals When a cation is formed from an atom of a transition metal, electrons are always removed first from the ns orbital and then from the (n – 1)d orbitals. Fe: [Ar]4 s 23 d 6 Mn: [Ar]4 s 23 d 5 Fe 2+: [Ar]4 s 03 d 6 or [Ar]3 d 6 Mn 2+: [Ar]4 s 03 d 5 or [Ar]3 d 5 Fe 3+: [Ar]4 s 03 d 5 or [Ar]3 d 5 35