Chemistry definition of PURE NOT PURE How do



























- Slides: 40



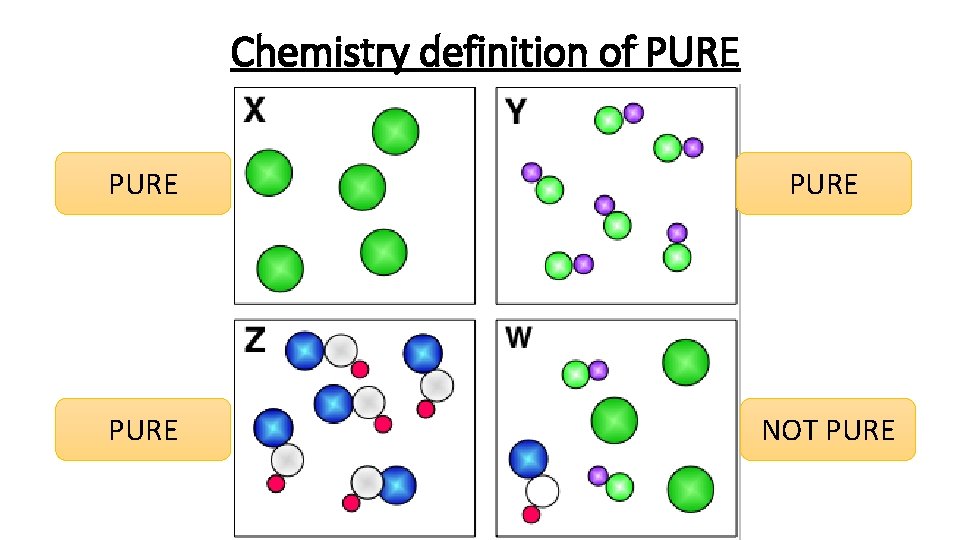
Chemistry definition of PURE NOT PURE

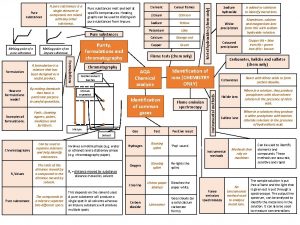
How do you know if something is pure? If an element or a compound is pure and not a mixture then it will melt or boil at a very specific temperature. We can use this information to find the purity of a substance. What temperature does pure water boil at? 100 °C!



Spec definition Formulations • A formulation is a mixture that has been designed as a useful product. Many products are complex mixtures in which each chemical has a particular purpose. • Formulations are made by mixing the components in carefully measured quantities to ensure that the product has the required properties. Formulations include fuels, cleaning agents, paints, medicines, alloys, fertilisers and foods.




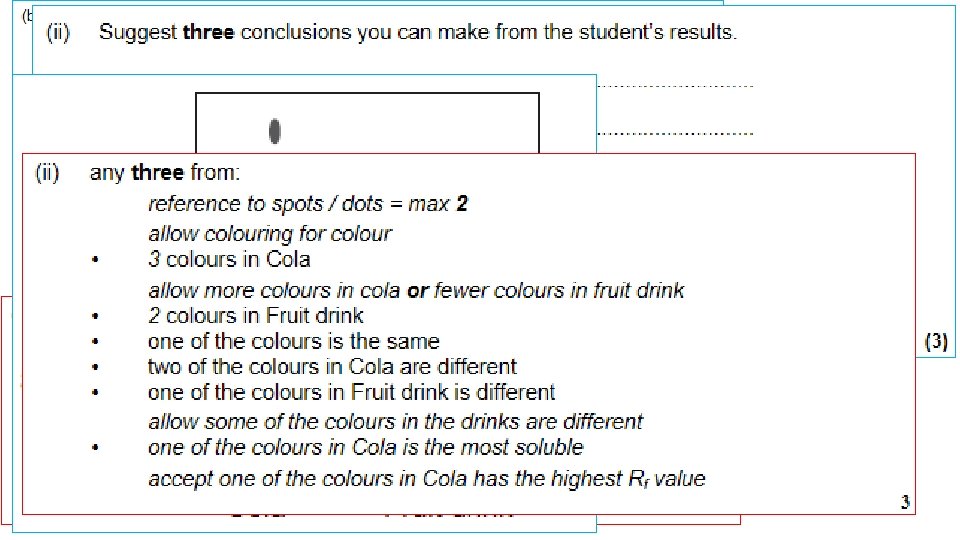
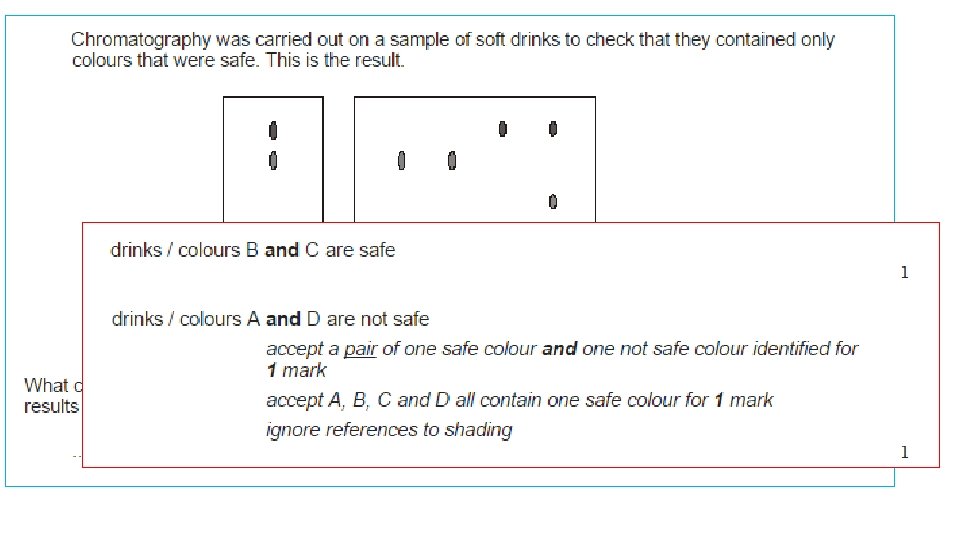
1. Draw a pencil line 1 cm up from the bottom of your chromatography paper. This is your baseline. 2. Spot four different inks along the baseline. 3. Secure the paper from the top and dip into your solvent, make sure the solvent does not go above the baseline. 4. Allow the solvent front (mobile phase), to travel up the paper (stationary phase). When it is one cm from the top, remove the paper from the solvent and allow to dry. 5. Use a pencil to draw around any marks on the paper and stick it to your page. 6. Conclude which colours the black ink is made from.


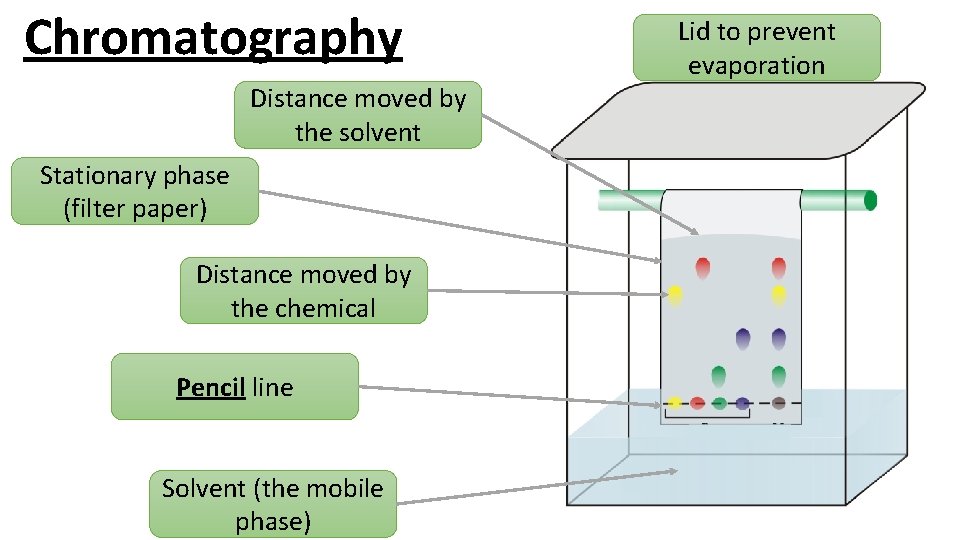
Chromatography Distance moved by the solvent Stationary phase (filter paper) Distance moved by the chemical Pencil line Solvent (the mobile phase) Lid to prevent evaporation








Retention factor (Rf) values Always measure to the MIDDLE of the spot The Rf factor is used to compare the components of various samples. The Rf values of suspect samples can be compared with known samples. Rf = distance from the base line to the spot distance from the base line to the solvent front If two substances have the same Rf value, they are likely (but not necessarily) the same compound. If they have different Rf values, they are definitely different compounds. Solvent front the point at which the water stopped moving up the paper Spot the point at which Base line a band or spot of the line where the original colour is sample was placed




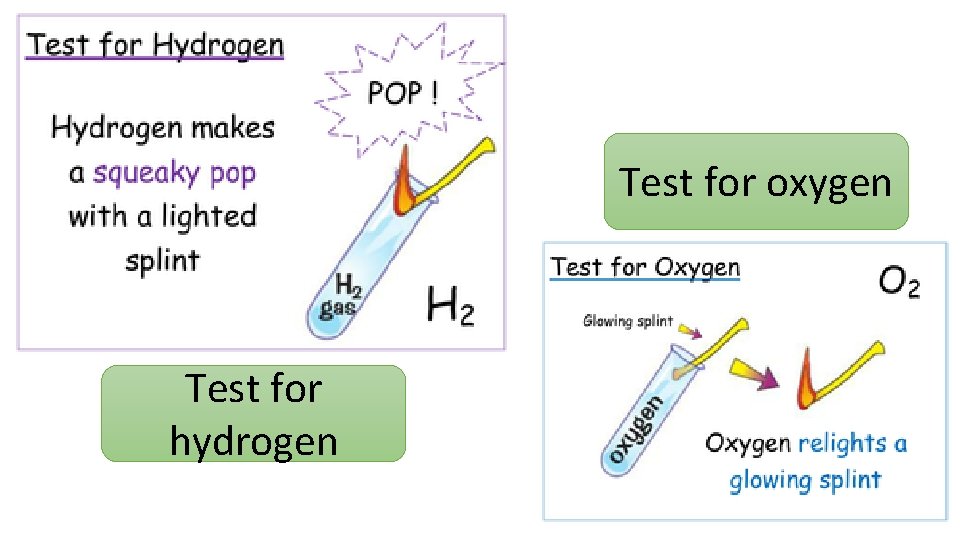
Test for oxygen Test for hydrogen


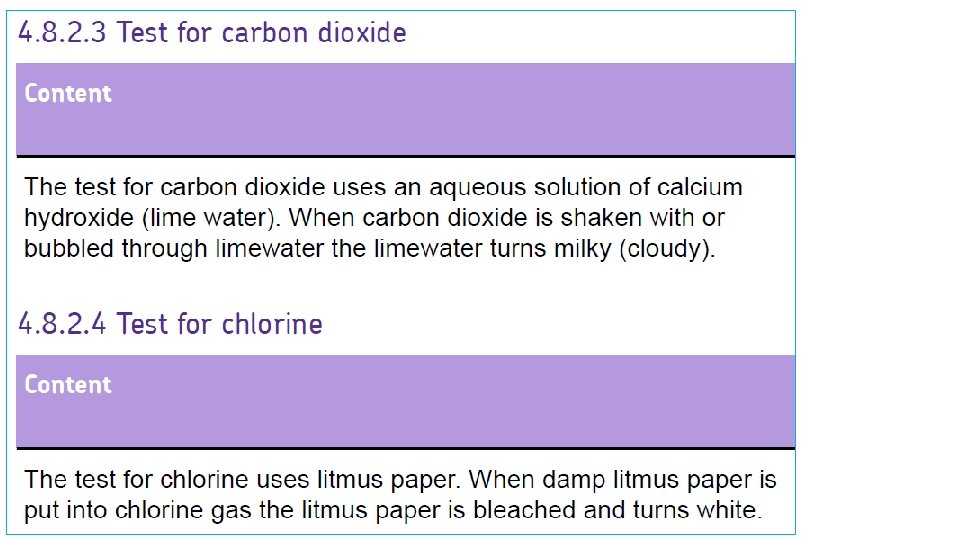
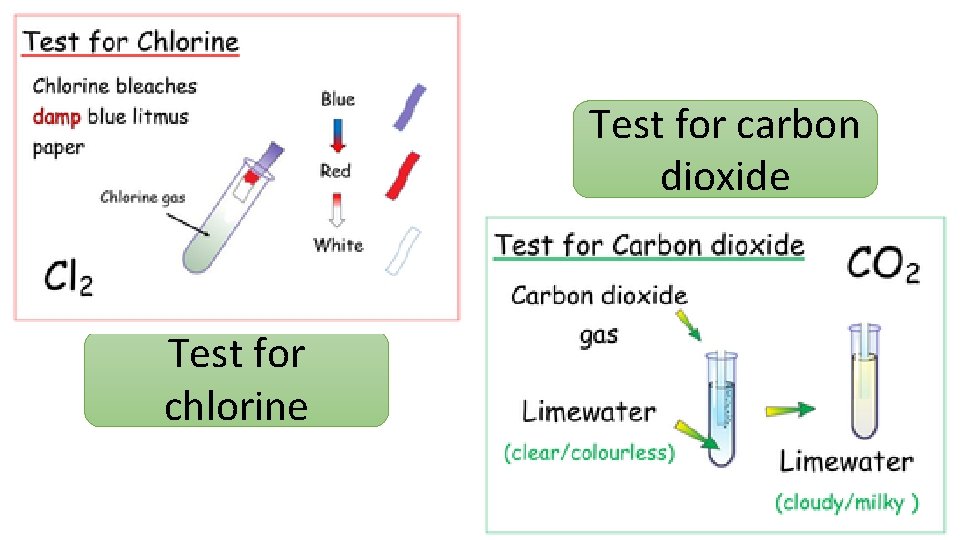
Test for carbon dioxide Test for chlorine

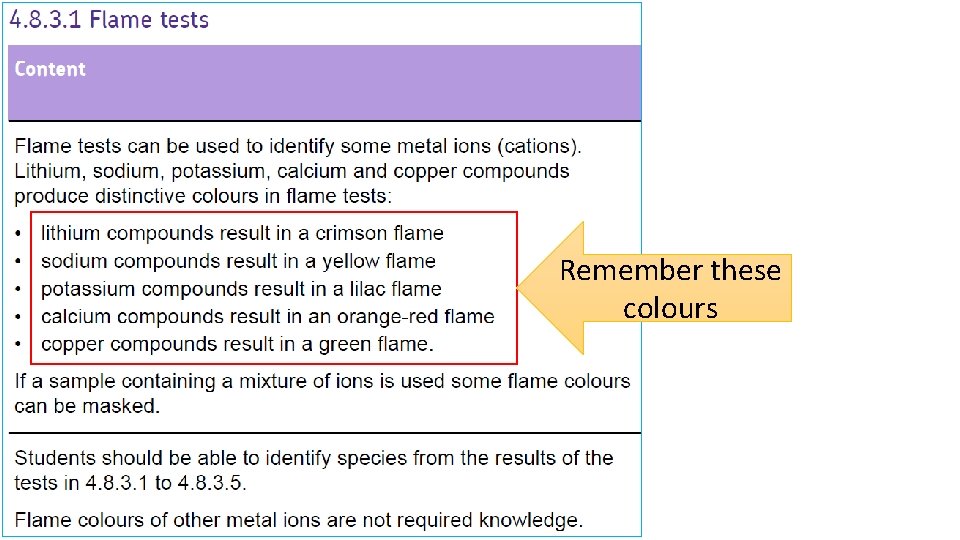
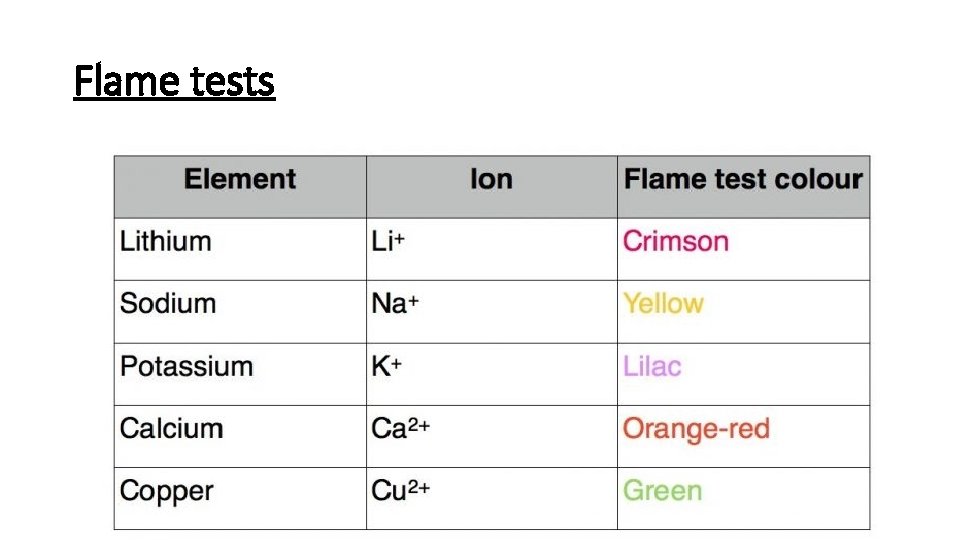
Remember these colours

Flame tests



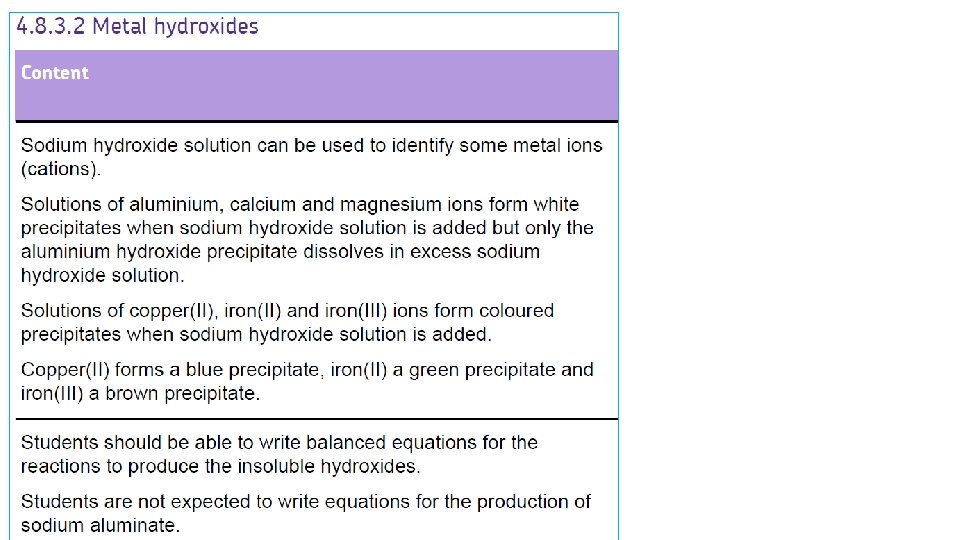
Testing for positive ions To test for positive ions, react the unknown substance with sodium hydroxide solution and observe the result Then flame test


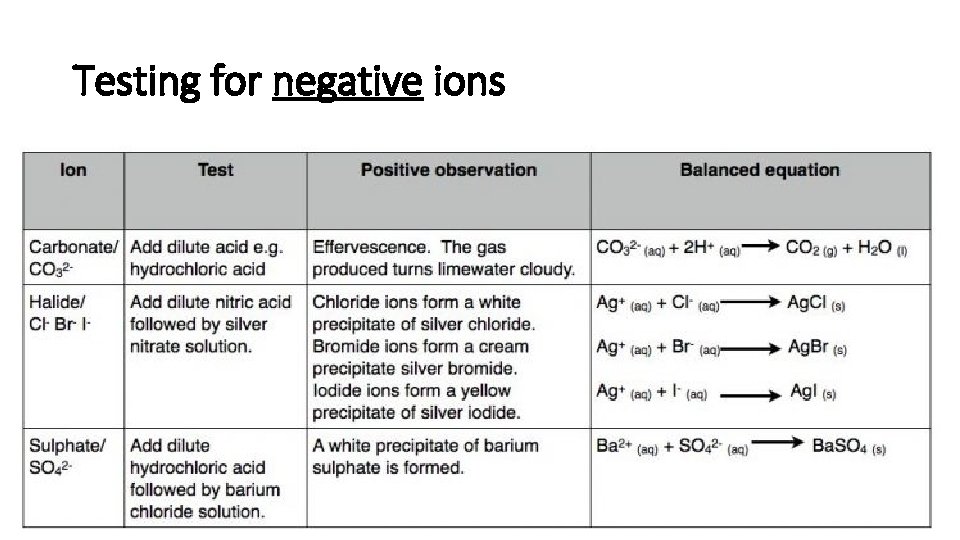
Testing for negative ions






 Definition of pure chemistry
Definition of pure chemistry Sudden and violent but brief; fitful; intermittent
Sudden and violent but brief; fitful; intermittent H=hf+xhfg
H=hf+xhfg Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Physical properties of a pure substance
Physical properties of a pure substance If you're not confused you're not paying attention
If you're not confused you're not paying attention Not dressy
Not dressy Attention is not explanation
Attention is not explanation Being too broad
Being too broad Negation of if
Negation of if Small just
Small just Love is not all you need
Love is not all you need Eyes that see and ears that hear
Eyes that see and ears that hear Pp sit
Pp sit If we can't measure it we can't manage it
If we can't measure it we can't manage it We will not be shaken we will not be moved
We will not be shaken we will not be moved Not a rustling leaf not a bird
Not a rustling leaf not a bird You can not not communicate
You can not not communicate Public goods
Public goods Goods definition
Goods definition What comes out of a man mouth is in his heart
What comes out of a man mouth is in his heart Pure substance examples
Pure substance examples Pure speech and symbolic speech
Pure speech and symbolic speech Pure bundling definition
Pure bundling definition Sublimation definition
Sublimation definition What is a pure discount loan
What is a pure discount loan Analytical chemistry definition
Analytical chemistry definition Molekülerite nedir
Molekülerite nedir Scientific notation definition
Scientific notation definition Define chemical potential
Define chemical potential Sublevel d
Sublevel d Nick the camel
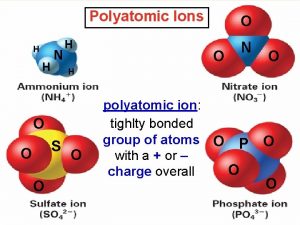
Nick the camel Example of polyatomic cation
Example of polyatomic cation Polarimetry definition in chemistry
Polarimetry definition in chemistry What is monograph
What is monograph Polyatomic compounds
Polyatomic compounds Define green chemistry
Define green chemistry Combustion formula example
Combustion formula example Dimensional analysis method
Dimensional analysis method Contraindications of composite restoration
Contraindications of composite restoration