Chemistry CP Metric Lab Reflection in NB How

Chemistry CP

Metric Lab Reflection in NB • How does the double stuff Oreo compare to the regular Oreo? Use numerical data from your third data table to support your answer.

Metric Lab Reflection 1. Make sure all three data tables have a title. • Data Table 1: Density Measurements 2. Make sure all data has 3 SF only and the correct units. • 1 cm= 1. 00 cm • 2. 345 g= 2. 35 g • 0. 8921 g= 0. 892 g Due by Thursday

How Do “Measure” Chemistry? • Metric System (International System of Units) • Dimensional Analysis • Significant Figures • Scientific Notation

Metric System International System of Units (SI) • • Length – meter (m) Mass – gram (g) Volume – liter (L or l) 1 2 Time – second (s) Temperature – Celsius (C) or Kelvin (K) 3 4 Amount of substance – Mole (mol) Energy- Joule (J)

Metric System Tools • Mass (g): gram scale • Length (m): Ruler and Meter Stick • Volume (L): Graduated Cylinder

Metric System Scale Multiple Big 1 Base Units (g, m, L) 1 3 1000 100 Thousand Hundred Kilo Hecto (k) (h) Small 2 0. 1 Tenth Deci (d) 10 Ten Deka (da) Division 4 0. 01 Hundredth Centi (c) 0. 001 Thousandth Milli (m)
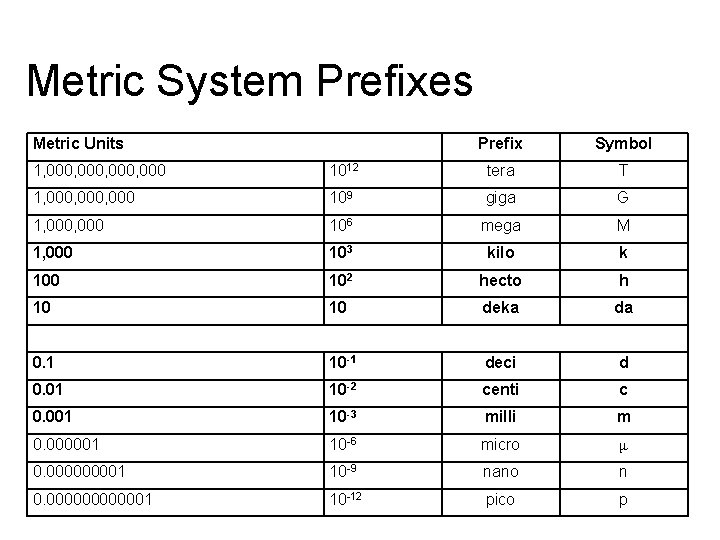
Metric System Prefixes Metric Units Prefix Symbol 1, 000, 000 1012 tera T 1, 000, 000 109 giga G 1, 000 106 mega M kilo k 1, 000 1 103 2 100 102 hecto h 10 10 deka da 3 4 0. 1 10 -1 deci d 0. 01 10 -2 centi c 0. 001 10 -3 milli m 0. 000001 10 -6 micro μ 0. 00001 10 -9 nano n 0. 0000001 10 -12 pico p
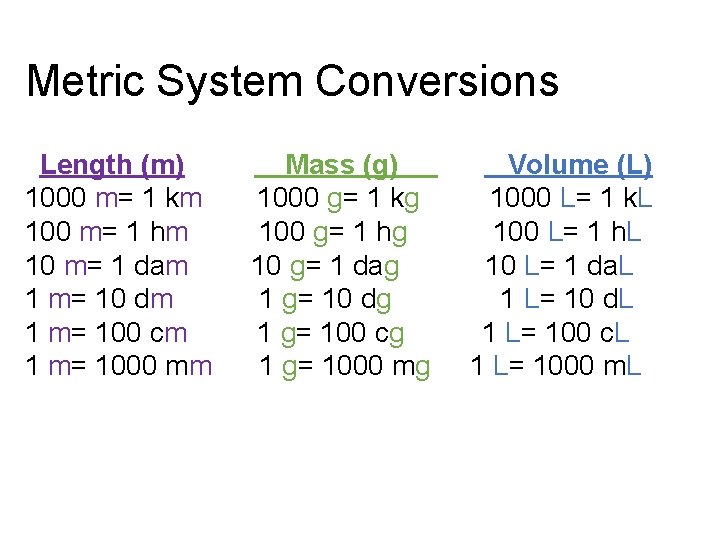
Metric System Conversions Length (m) 1000 m= 1 km 100 m= 1 hm 10 m= 1 dam 1 m= 10 dm 1 m= 100 cm 1 m= 1000 mm Mass (g) 1000 g= 1 kg 100 g= 1 hg 10 g= 1 dag 1 g= 10 dg 1 g= 100 cg 1 g= 1000 mg Volume (L) 1000 L= 1 k. L 100 L= 1 h. L 10 L= 1 da. L 1 L= 10 d. L 1 L= 100 c. L 1 L= 1000 m. L

How Do “Measure” Chemistry? • Metric System (International System of Units) • Dimensional Analysis (Conversion Factors) • Significant Figures • Scientific Notation

Dimensional Analysis (2 Step) If you do not have a conversion factor between the given unit and the one you are looking for, you must do two conversions. How many milliliters are in 0. 005 kiloliters?
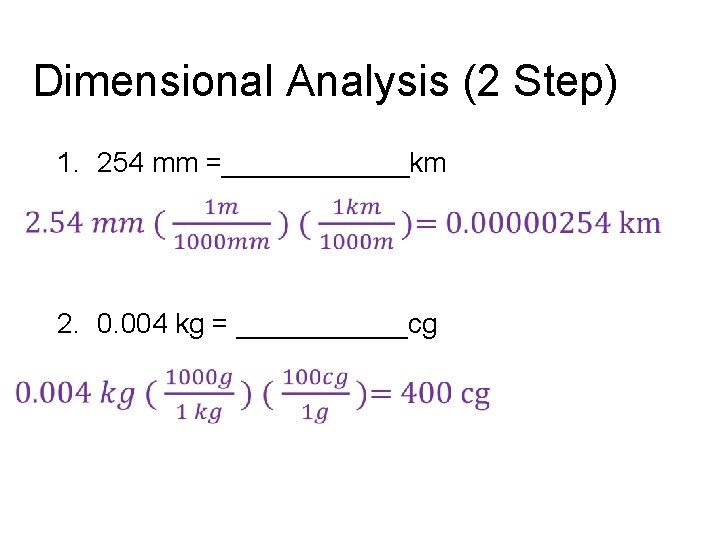
Dimensional Analysis (2 Step) 1. 254 mm =______km 2. 0. 004 kg = ______cg
How Do “Measure” Chemistry? • Metric System (International System of Units) • Dimensional Analysis • Significant Figures • Scientific Notation
Significant Figures Rules 1. Non-zero digits are always significant. 26. 38= 4 SF 2. Any zeros between two significant digits are significant. (Captured Zeros) 406= 3 SF 91, 600= 3 SF 3. A final zero or trailing zeros in the decimal portion ONLY are significant. 0. 00500= 3 SF 0. 03040= 4 SF
Significant Figures Rounding Practice in NB Express the following using 3 significant figures: 1. 600 (1 SF) 600. (3 SF) 2. 326451 (6 SF) 326000 or 3. 26 x 105 (3 SF) 3. 0. 000004555 (4 SF) 0. 00000456 or 4. 56 x 10 -6 (3 SF) 4. 3995 (4 SF) 4. 00 x 103 (3 SF) 5. 48000 (2 SF) 4. 80 x 104 (3 SF)
Significant Figure Rules Continued… Multiplying / Dividing / Trigonometric Functions 1. Perform all the operations 2. Round off the result so that it has the same number of sig figs as the least of all those used in your calculation. (2. 5 m) x (2. 01 m) x (2. 755 m) = 13. 843875 m Answer = 14 m 3 (2 sig figs)

Significant Figure Rules Continued… Adding/ Subtracting 1. Perform all the operations 2. The answer can contain no more decimal places than the least accurate measurement. 153. ml + 1. 8 ml + 9. 16 ml = 163. 96 ml Answer = 164 ml
How Do “Measure” Chemistry? • Metric System (International System of Units) • Dimensional Analysis • Significant Figures • Scientific Notation
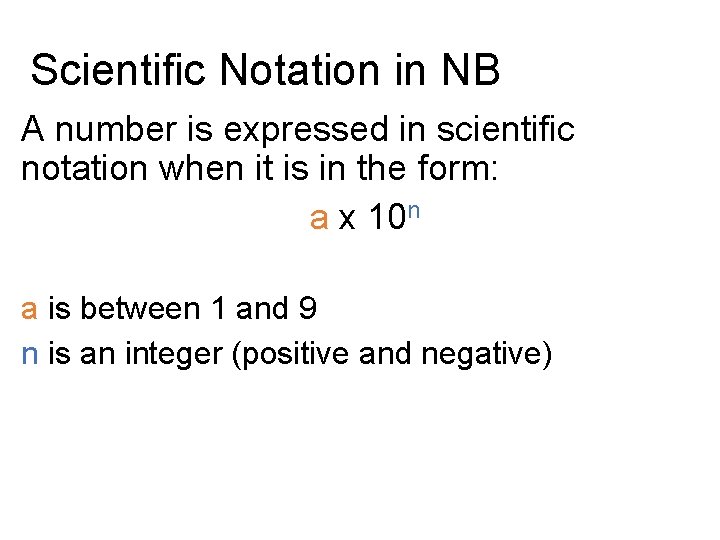
Scientific Notation in NB A number is expressed in scientific notation when it is in the form: a x 10 n a is between 1 and 9 n is an integer (positive and negative)

Scientific Notation 210, 000, 000, 000 1. Where is the decimal point now? After the last zero 2. Where would you put the decimal to make this number be between 1 and 9? Between the 2 and the 1 3. How many decimal places did you move the decimal? 23 4. When the original number is more than 1, the exponent is positive. 2. 1 x 1023

Scientific Notation 0. 0000000902 1. Where would the decimal go to make the number be between 1 and 9? 9. 02 2. The decimal was moved how many places? 8 3. When the original number is less than 1, the exponent is negative. 9. 02 x 10 -8
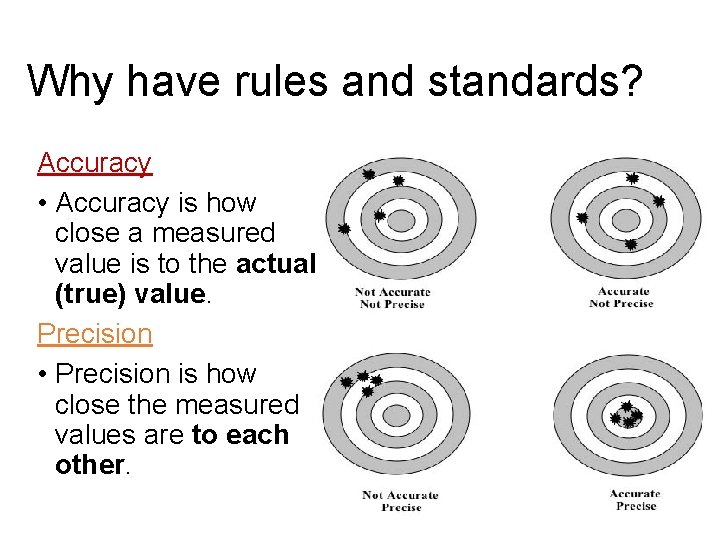
Why have rules and standards? Accuracy • Accuracy is how close a measured value is to the actual (true) value. Precision • Precision is how close the measured values are to each other.

Homework • • Metric System and Scientific Notation 8 -26 Quiz on 8 -27 1 2 3 4
- Slides: 23