Chemistry CP Measurements and Calculations Chapter 2 Scientific








































- Slides: 60

Chemistry CP Measurements and Calculations Chapter 2

Scientific Method Organized plan for gathering, organizing, and communicating information ✦ 1. Define a Problem ✦ 2. Make Observations ✦ 3. Make a Hypothesis ✦ 4. Test your Hypothesis - Experiment ✦ 5. Collect Data ✦ 6. Form a Conclusion

Making Observations ✦ Observations lead to making measurements and collecting data ✦ Qualitative data - descriptive ✦ ✦ Ex. - the sky is blue Quantitative data - numerical ✦ Ex. - 115. 3 m. L of H 2 O


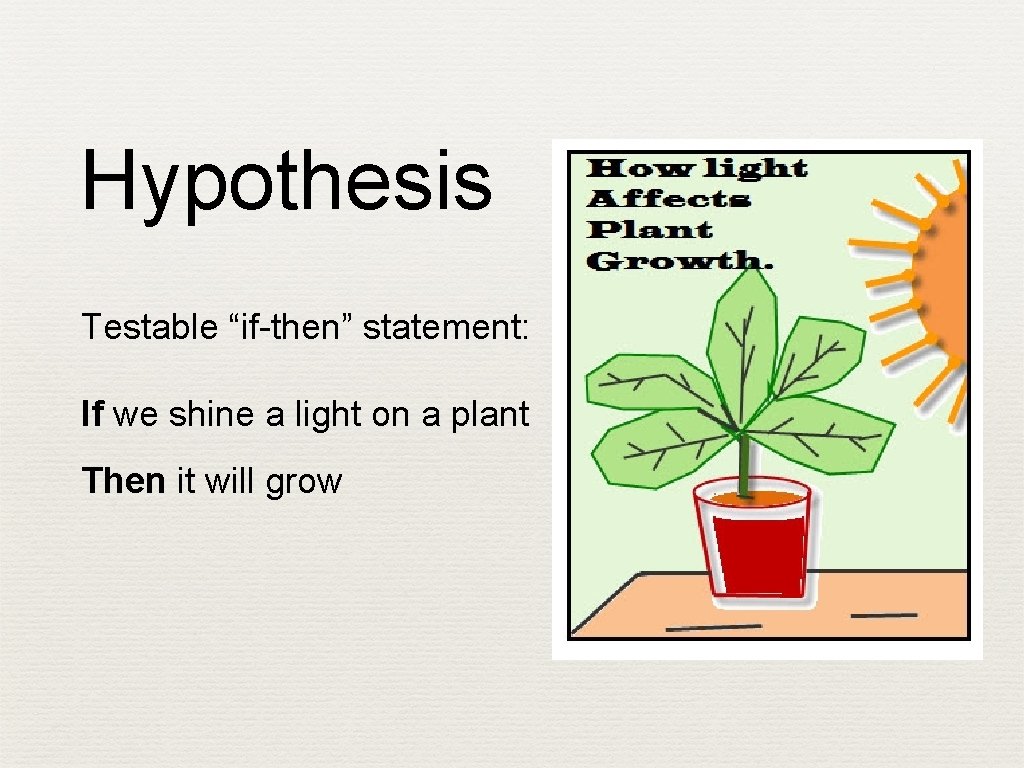
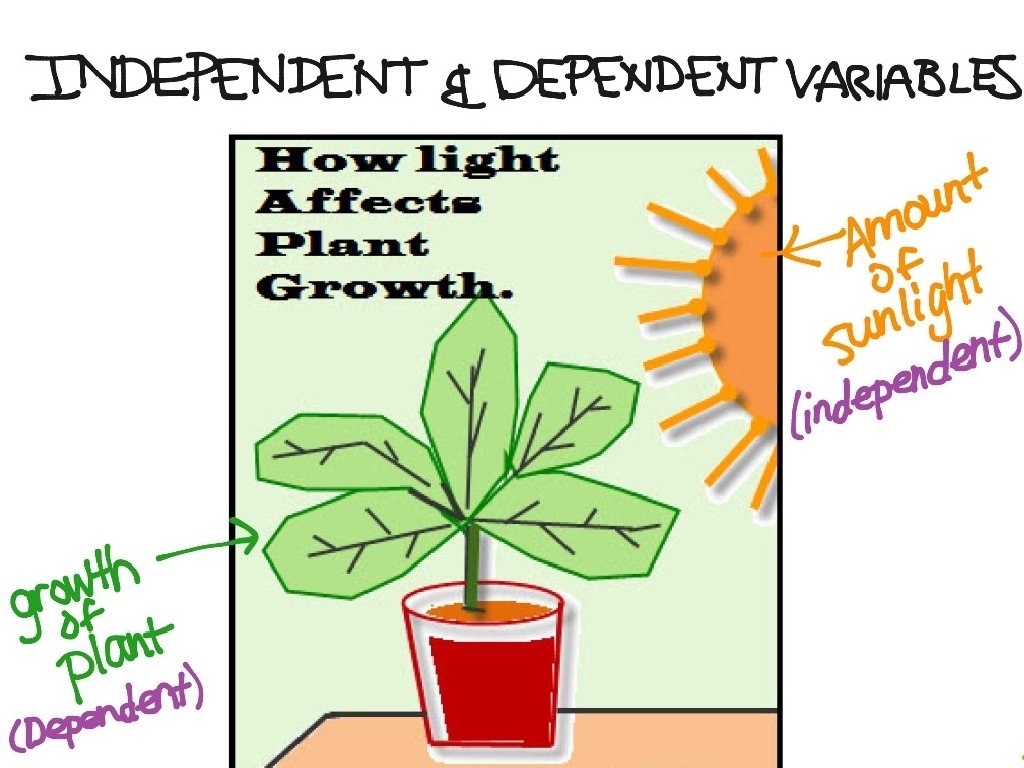
Hypothesis Testable “if-then” statement: If we shine a light on a plant Then it will grow

Experiment Organized procedure for testing a hypothesis Control - conditions that remain constant, standard for comparison Variable - any condition that changes

✦ Two Types of Variables Independent variable - controlling this variable causes change in the other variable ✦ ✦ Ex: Time Dependent variable - variable that changes in response to the independent variable ✦ Ex: Temperature


Theory ✦ Scientific explanation for observations repeatedly verified over a long period of time ✦ ✦ ✦ Broad generalization that explains a body of facts or phenomena Theories accurately predict results in many experiments Most theories cannot be disproved and are still being tested for validity ✦ Ex: Theory of Relativity

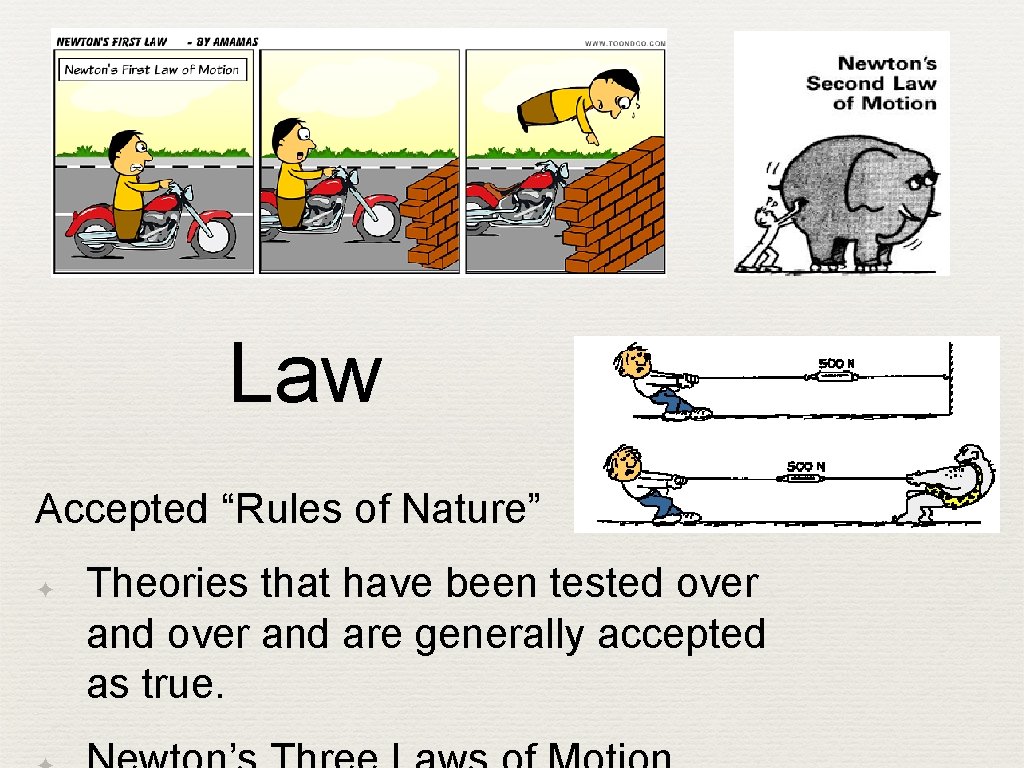
Law Accepted “Rules of Nature” ✦ Theories that have been tested over and are generally accepted as true.

Units of Measurement ✦ ✦ Quantity – something that has magnitude, size, or amount Unit – comparison of what is measured with a previously defined size. Ex: Meter

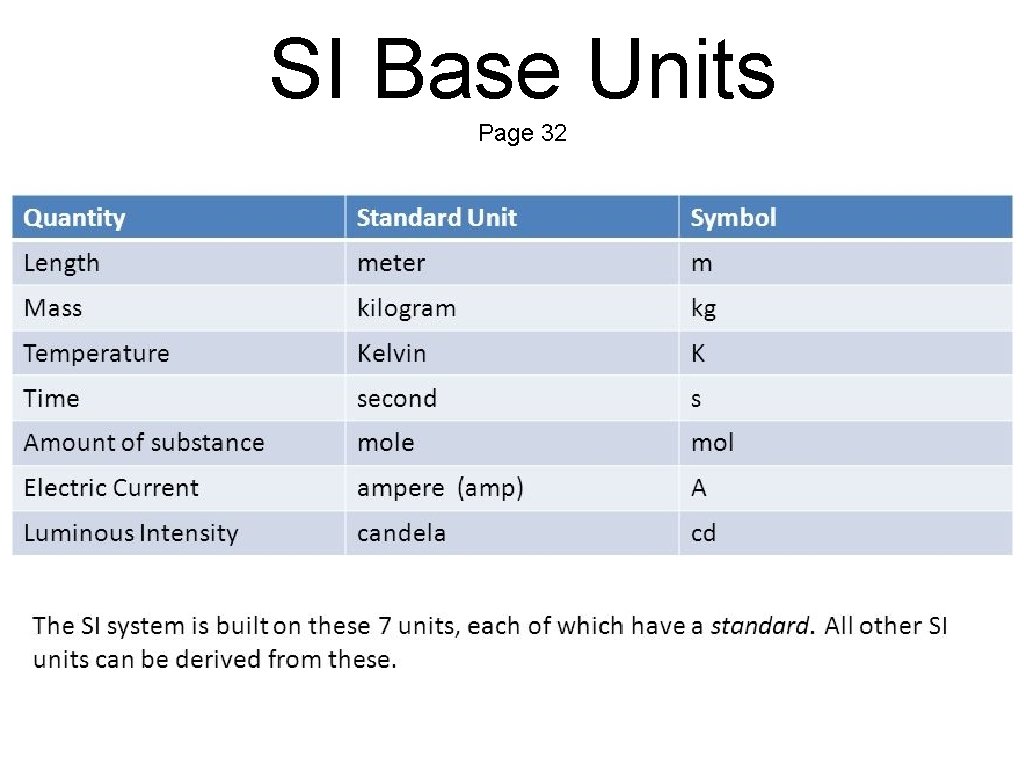
SI Base Units Page 32

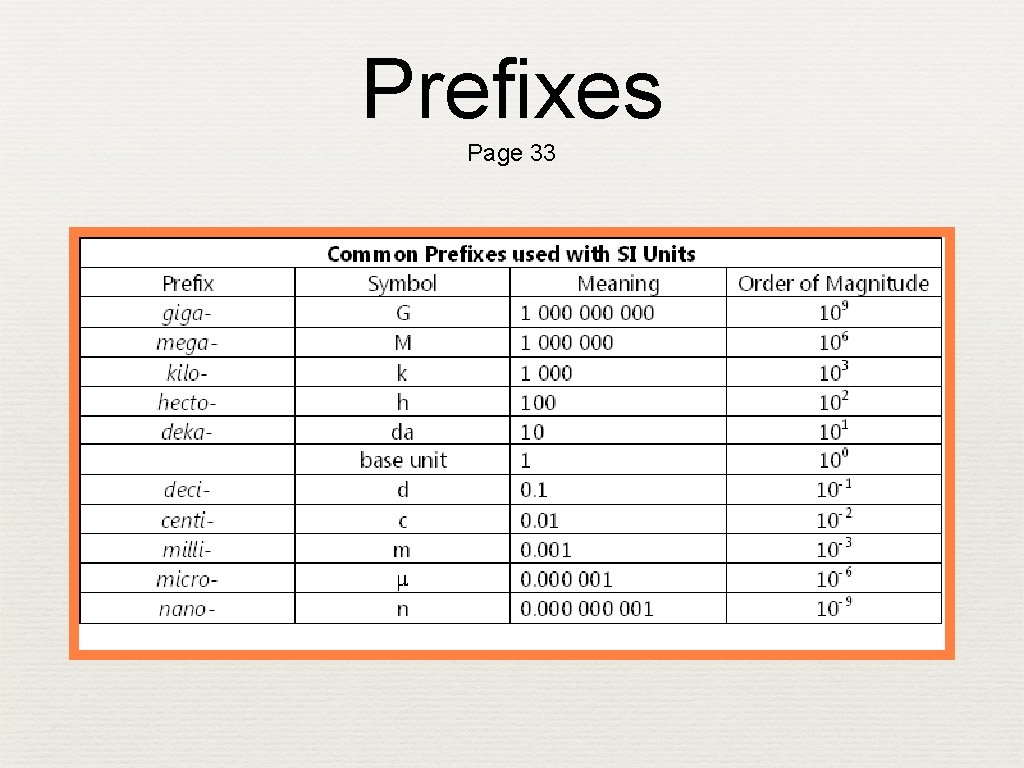
Prefixes Page 33

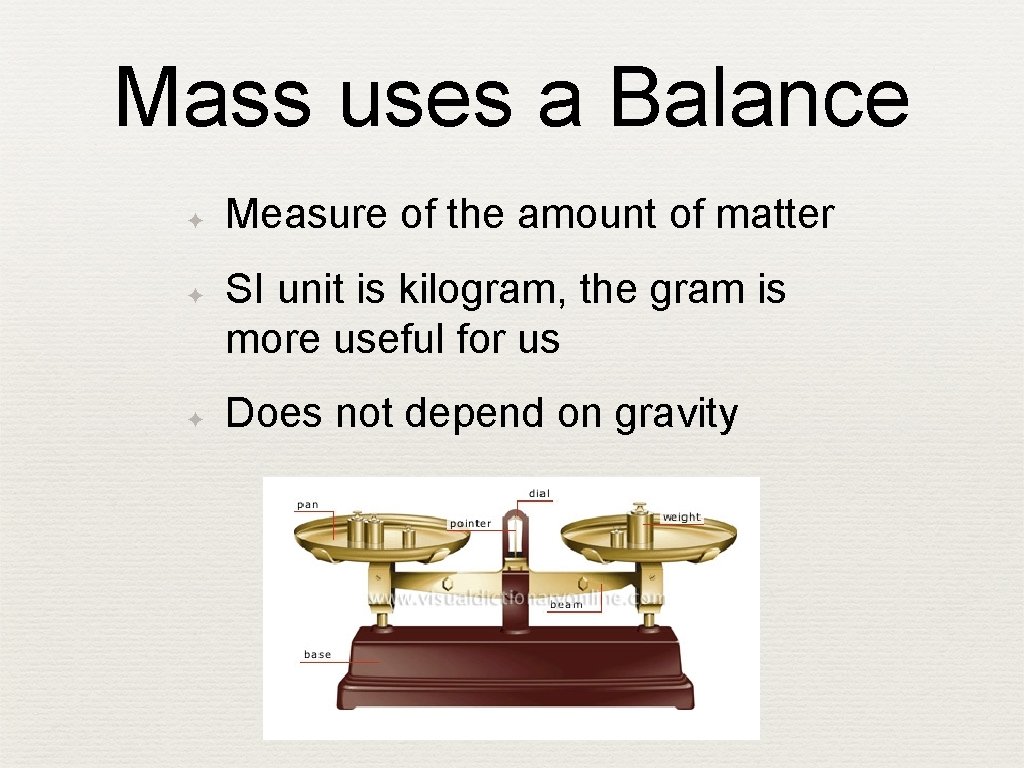
Mass uses a Balance ✦ ✦ ✦ Measure of the amount of matter SI unit is kilogram, the gram is more useful for us Does not depend on gravity

Weight uses a Scale ✦ ✦ Weight - measure of the gravitational pull on matter The astronaut shown on the surface of the moon weighs one sixth of what he weighs on Earth.

Length ✦ ✦ Used to measure distance of an object SI unit is the meter, longer distances use the kilometer

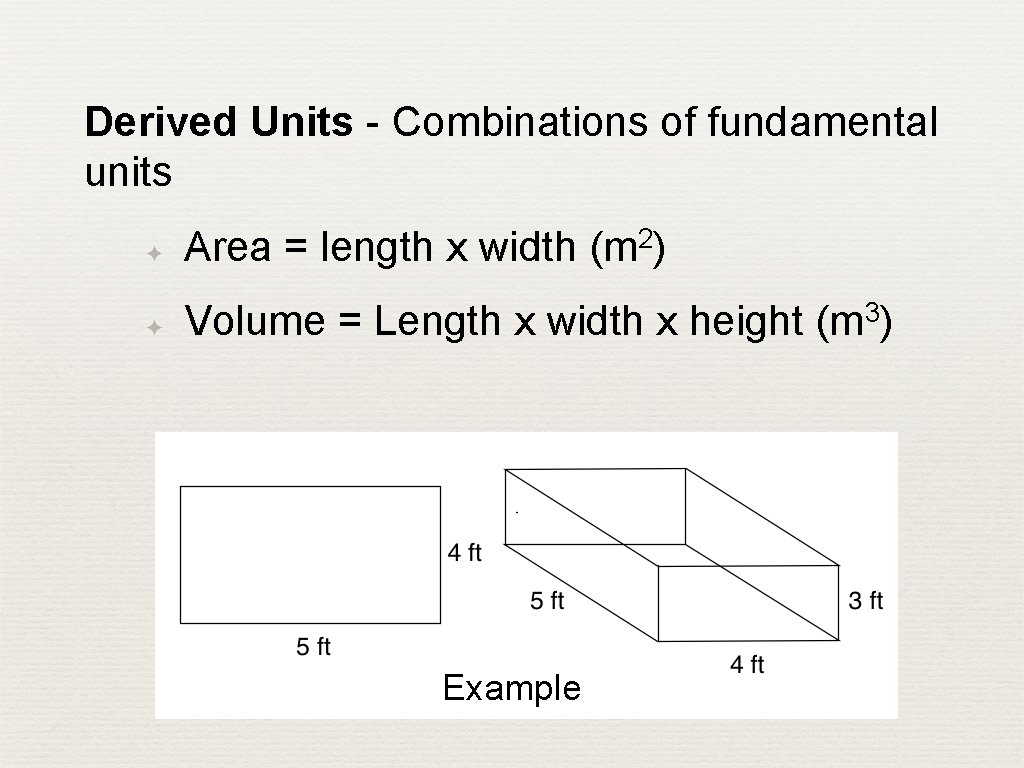
Derived Units - Combinations of fundamental units ✦ 2 Area = length x width (m ) ✦ Volume = Length x width x height (m 3) Example

Volume ✦ Amount of space occupied by an object ✦ SI unit - m 3 ✦ Smaller unit, cm 3 is typically used ✦ Liquid volumes use the non-SI unit, liter ✦ Irregularly shaped objects use displacement to find volume

Comparing Volumes ✦ 1 liter = 1 dm 3 ✦ 1 liter = 1000 m. L ✦ 1 m. L = 1 cm 3 ✦ Therefore, 1 liter = 1000 cm 3

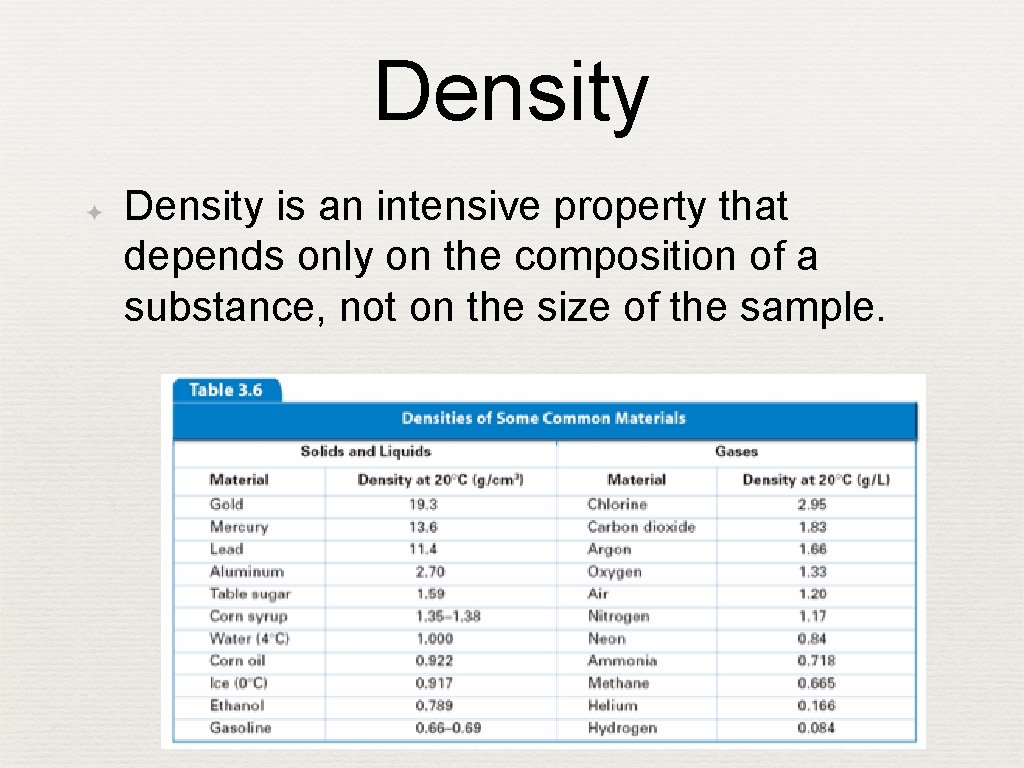
Density ✦ Density is an intensive property that depends only on the composition of a substance, not on the size of the sample.

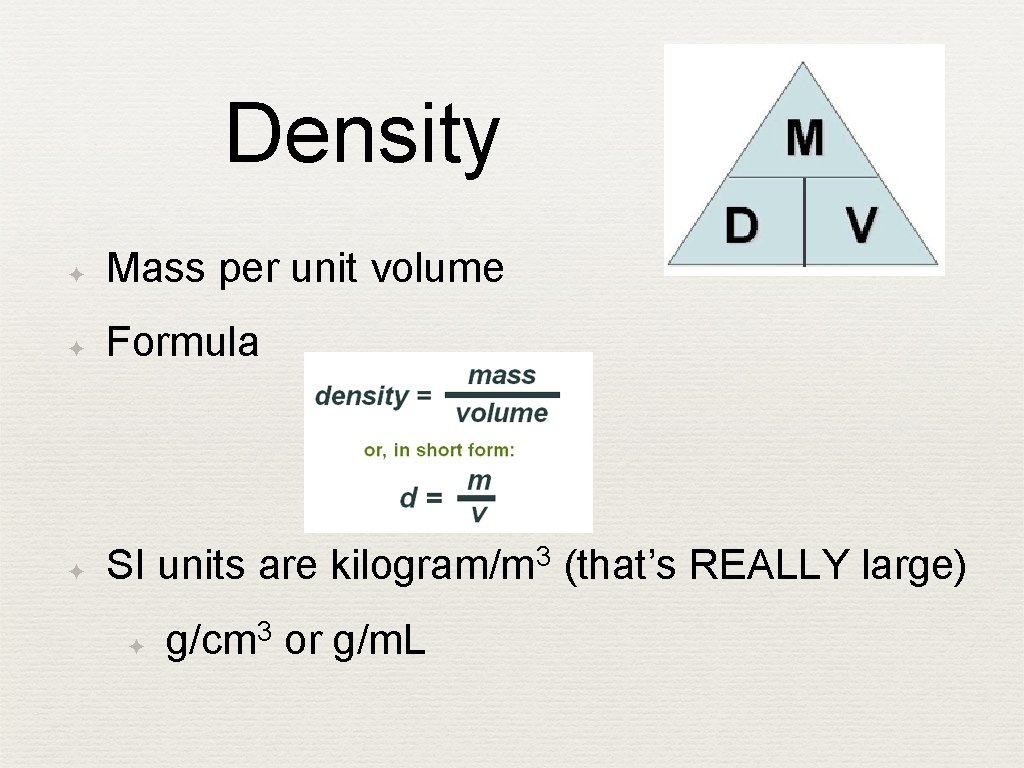
Density ✦ Mass per unit volume ✦ Formula ✦ SI units are kilogram/m 3 (that’s REALLY large) ✦ g/cm 3 or g/m. L

Density and Temperature ✦ ✦ ✦ Does temperature affect density? Density generally decreases as temperature increases Why? Typically the volume of most substances increases as the temperature increases, but mass remains the same. Thus, the density must change. How does that look mathematically?

Density Examples ✦ A copper penny has a mass of 3. 1 g and a volume of 0. 35 cm 3. What is the density of copper?

Density Examples ✦ What is the volume of a pure silver coin that has a mass of 14 g? The density of silver 3 (Ag) is 10. 5 g/cm.

Density Examples ✦ The density of manganese, a metallic element is 7. 21 g/cm 3. What is the density in kg/m 3?

Specific Gravity ✦ ✦ Comparison of the density of a substance to the density of a reference substance, (usually water), at the same temperature No units

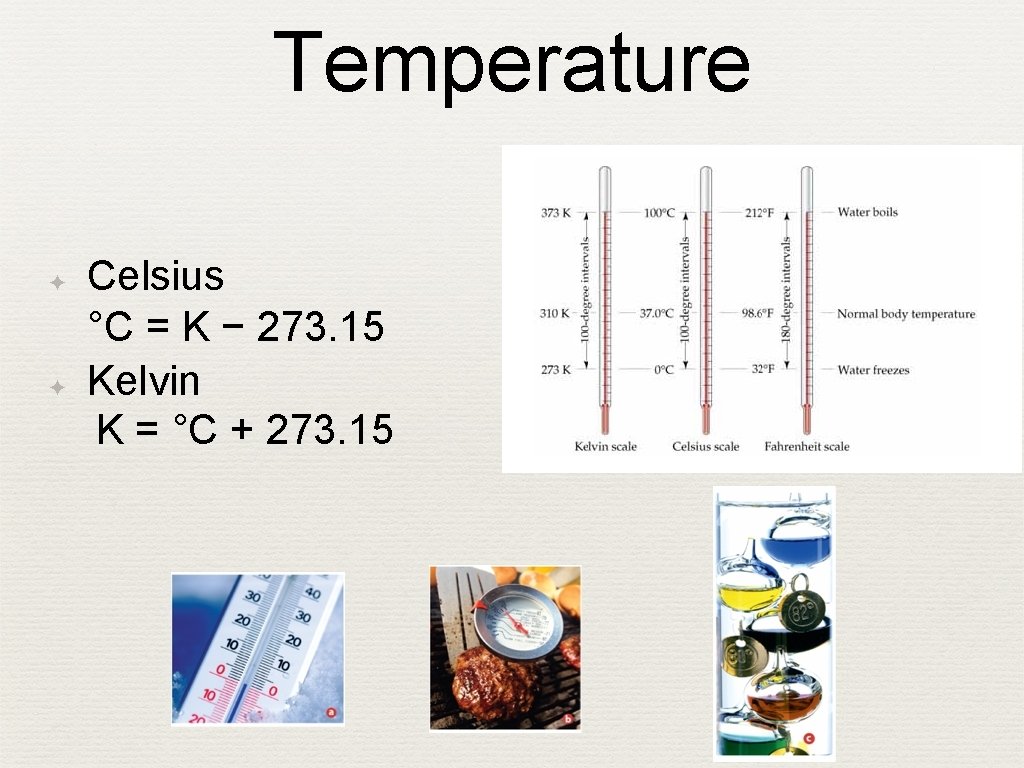
Temperature ✦ ✦ Celsius °C = K − 273. 15 Kelvin K = °C + 273. 15 27

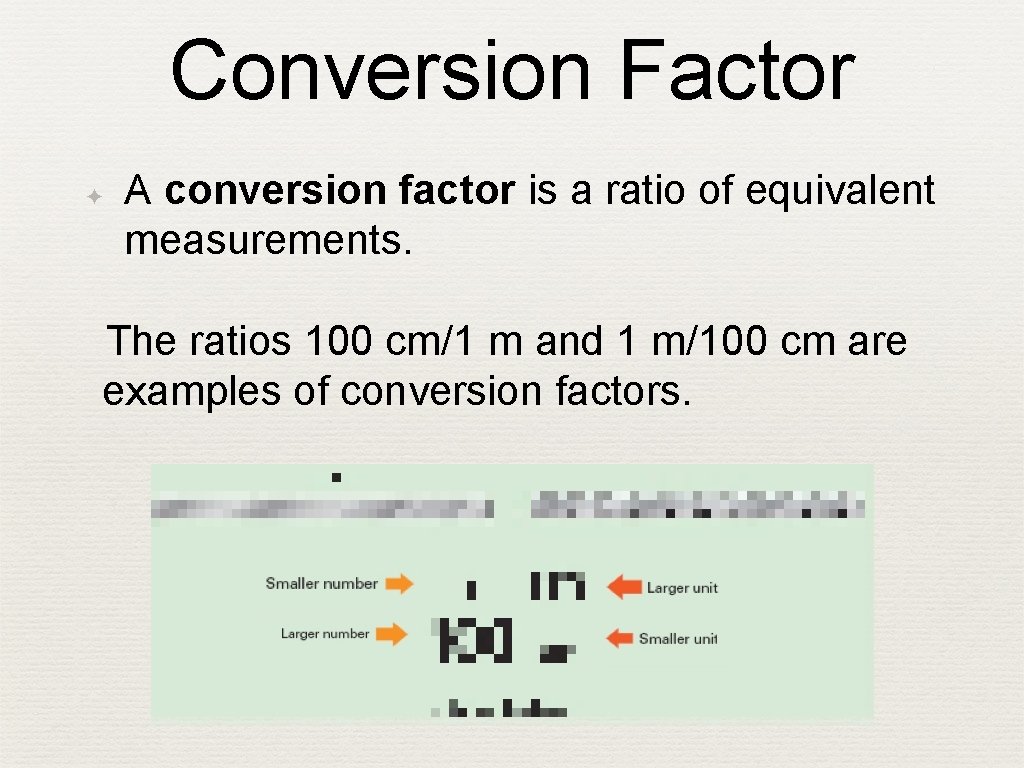
Conversion Factor ✦ A conversion factor is a ratio of equivalent measurements. The ratios 100 cm/1 m and 1 m/100 cm are examples of conversion factors.

2 -3 Using Scientific Measurements 1/5 the width of a human hair 2. 7 microns tall Human hair 75 microns thick 1 mm = 1000 microns, 1 m = 1000000 microns

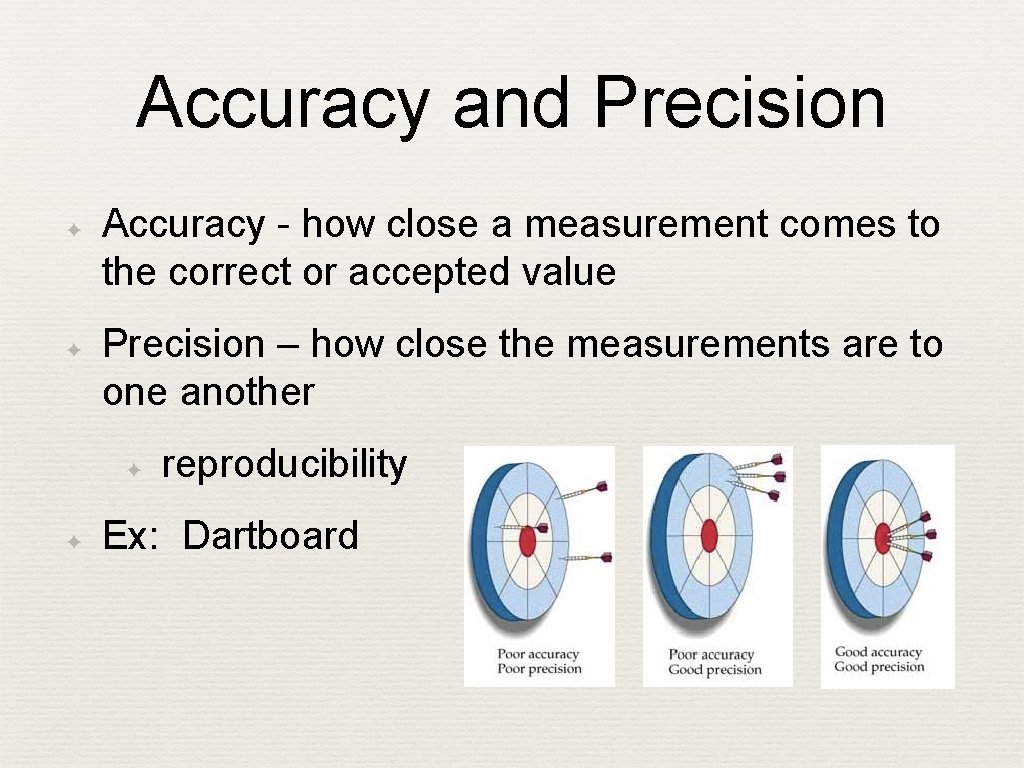
Accuracy and Precision ✦ ✦ Accuracy - how close a measurement comes to the correct or accepted value Precision – how close the measurements are to one another ✦ ✦ reproducibility Ex: Dartboard

Clicker Question Which set of measurements of a 2. 00 -g standard is the most precise? A. 2. 00 g, 2. 01 g, 1. 98 g B. 2. 10 g, 2. 00 g, 2. 20 g C. 2. 02 g, 2. 03 g, 2. 04 g D. 1. 50 g, 2. 00 g, 2. 50 g

✦ Percent Error – How far away from the accepted value are you? ✦ ✦ Value experimental - Valueaccepted % error = x 100 Valueaccepted Negative % error means that the experimental value is less than the accepted value Positive % error means that the experimental value is greater than the accepted value

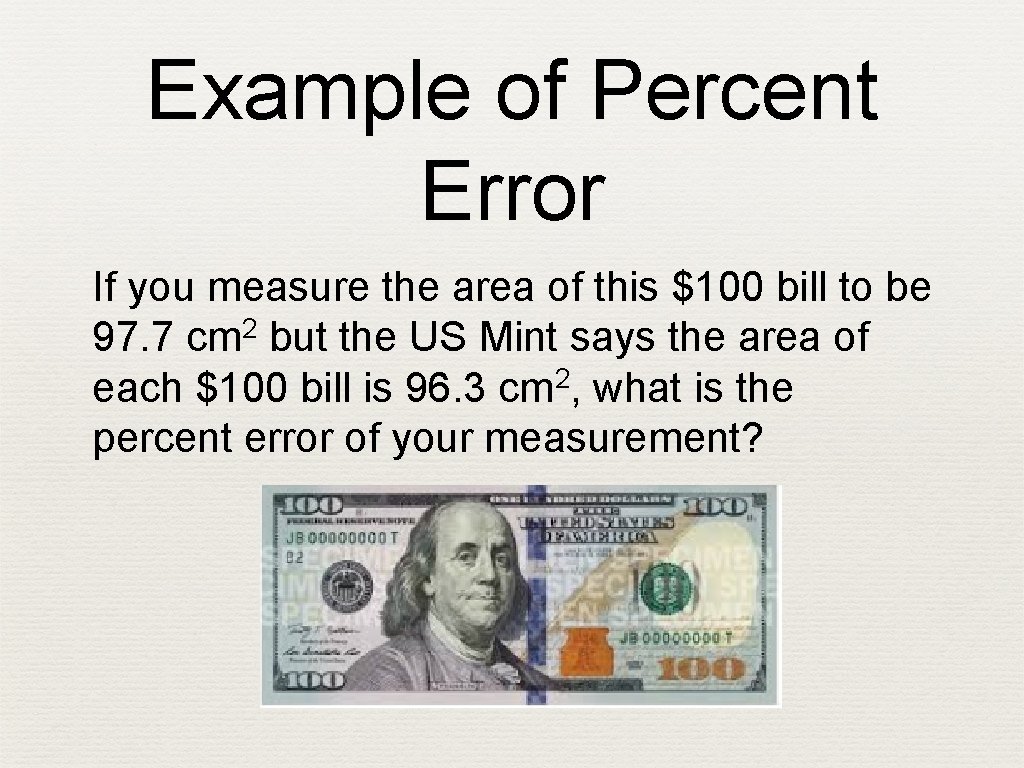
Example of Percent Error If you measure the area of this $100 bill to be 97. 7 cm 2 but the US Mint says the area of each $100 bill is 96. 3 cm 2, what is the percent error of your measurement?

✦ ✦ Just because a device works, don’t presume it’s accurate. The scale below is not properly zeroed, so the weight is inaccurate. What is the percent error of a measured value of 114 lbs if the person’s actual weight is 107 lbs?

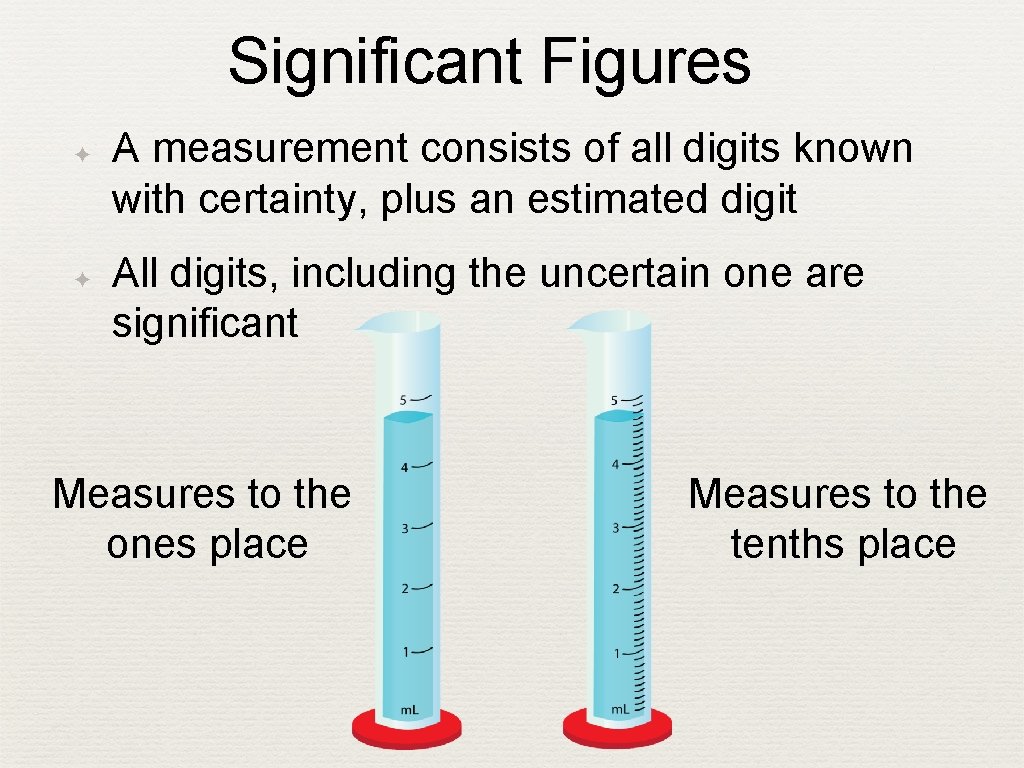
Significant Figures ✦ ✦ A measurement consists of all digits known with certainty, plus an estimated digit All digits, including the uncertain one are significant Measures to the ones place Measures to the tenths place

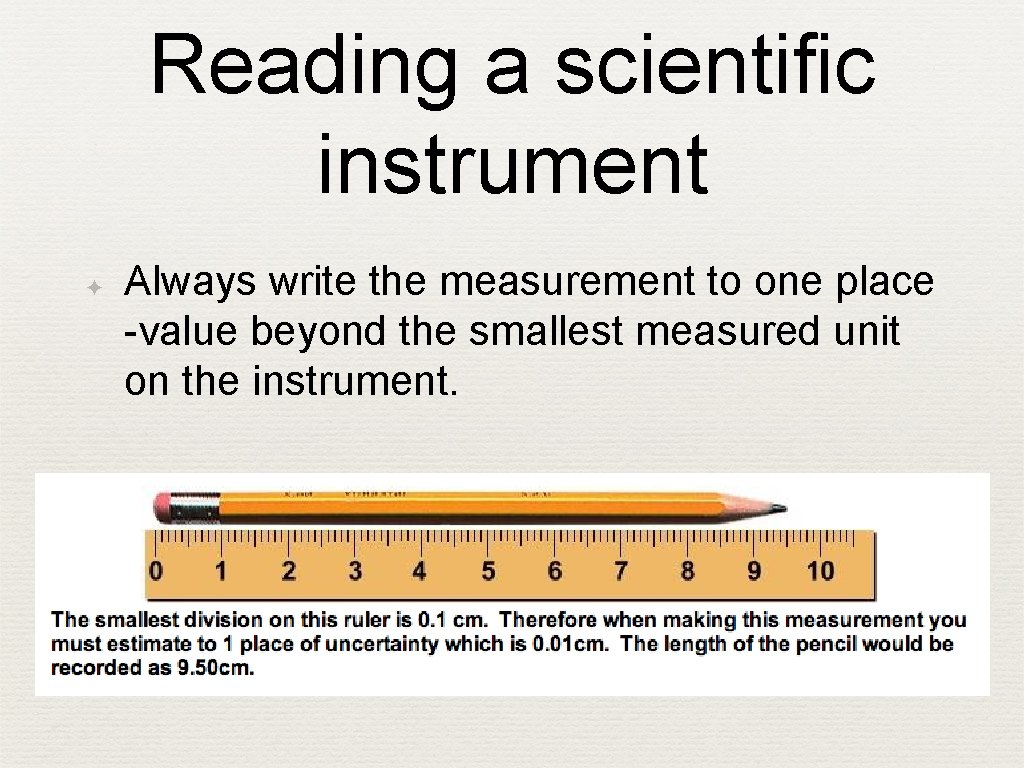
Reading a scientific instrument ✦ Always write the measurement to one place -value beyond the smallest measured unit on the instrument.

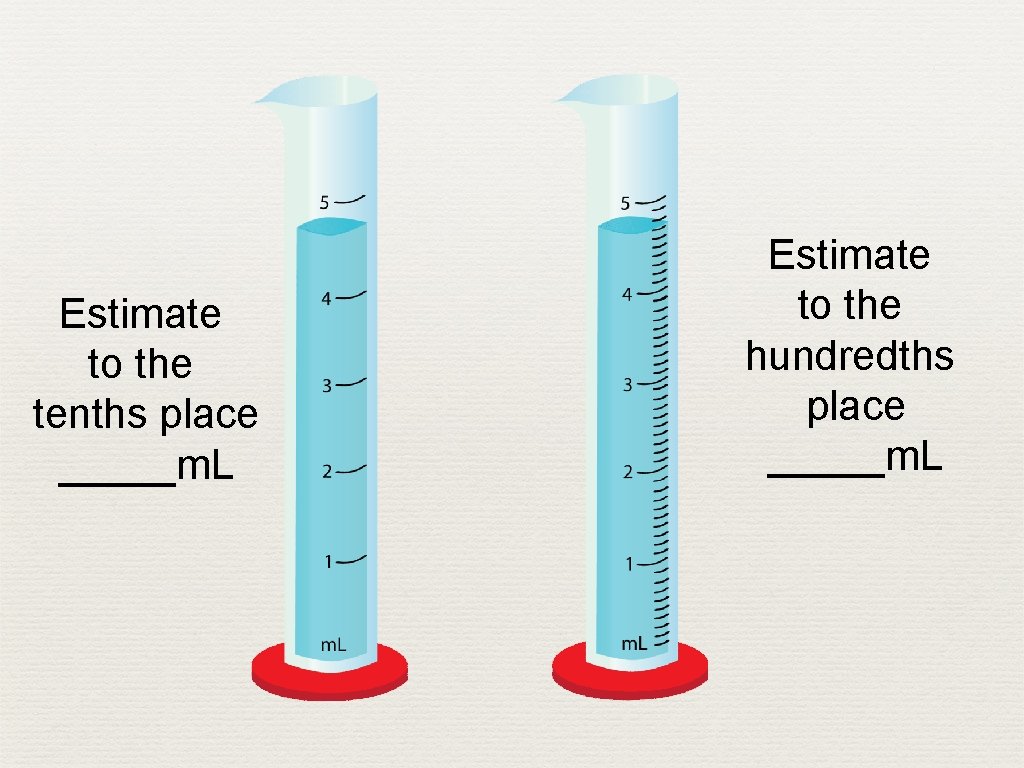
Estimate to the tenths place _____m. L Estimate to the hundredths place _____m. L

Identifying Significant Figures ✦ ✦ ✦ Every non zero digit is significant All zeros between nonzero digits are significant All zeros in front of nonzero digits are NOT significant ✦ Examples ✦ ✦ ✦ 0. 0025 0. 000009 0. 00900412 8 ✦ 2 sig figs ✦ 1 sig fig ✦ 7 sig figs

Zeros at the end ✦ And to the right of a decimal point are significant ✦ ✦ Are significant in measurements that have a written decimal point ✦ ✦ Ex: 107. 0 4 sig figs Ex: 540 000. 6 sig figs Are NOT significant if they are placeholders ✦ Ex: 540 000 2 sig figs

Examples of Sig Figs ✦ How many significant figures are in the following numbers ✦ 4 ✦ 3. 9 ✦ 0. 0056040

Significant Figures in Calculations ✦ Addition and subtraction ✦ ✦ Answers can only have the same number of decimal places as the measurement with the fewest Multiplication and Division ✦ Answers can only have the same number of sig figs as the measurement with the fewest

Examples of Sig Figs ✦ Calculations with Sig Figs ✦ 13. 64 + 0. 075 + 67 =81 ✦ 267. 8 - 9. 36 = 258. 4 ✦ 3021. 25 + 2105 =5126

Examples of Sig Figs ✦ Calculations with Sig Figs ✦ 3 608. 3 x 3. 45 =2. 10 x 10 ✦ 4. 8 / 392 = 0. 012

One Last Thing ✦ Counting numbers have an infinite number of sig figs ✦ Example: 10 test tubes ✦ There is NO uncertainty

Clicker Question A student reports the volume of a liquid as 0. 0130 L. How many significant figures are in this measurement? A. 2 B. 3 C. 4 D. 5

✦ Scientific Notation M x 10 ✦ M = number greater than 1 and less than 10 ✦ ✦ e e = exponent indicating the number of positions the decimal must be moved to achieve M If you move the decimal to the left e is positive If you move the decimal to the right e is negative Ex: 65 000 = 6. 5 x 10 -4 4 10 0. 000345 = 3. 45 x

✦ ✦ ✦ Addition and Subtraction - numbers can be added or subtracted if the powers of 10 are equal always retain the same power of 10 in your answer Ex : 5 x 103 + 4. 0 x 104 4 4. 5 x 10 0. 5 x 104 + 4. 0 x 104 =

✦ ✦ Multiplication - multiply the two numbers before the power of 10 - then add the exponents 7 6 x 10 5 2 Ex - (2 x 10 )(3 x 10 ) = Division - Divide the two numbers before the power of 10 - then subtract the exponents 7. 5 x 105 2. 5 x 103 Ex - = 3. 0 x 102

Energy ✦ ✦ Energy is the capacity to do work or to produce heat. The joule and the calorie are common units of energy. The joule (J) is the SI unit of energy. One calorie (cal) is the quantity of heat that raises the temperature of 1 g of pure water by 1°C. ✦ 1 J = 0. 2390 cal or 1 cal = 4. 184 J 49

Clicker Question ✦ In which of the following expressions is the number on the left NOT equal to the number on the right? A. 0. 00456 x 10– 8 = 4. 56 x 10– 11 B. 454 x 10– 8 = 4. 54 x 10– 6 C. 842. 6 x 104 = 8. 426 x 106 D. 0. 00452 x 106 = 4. 52 x 109

Clicker Question Which of the following is not a base SI unit? ✦ A. meter ✦ B. gram ✦ C. second ✦ D. mole

Clicker Question If you measured both the mass and weight of an object on Earth and on the moon, you would find that A. both the mass and the weight do not change. B. both the mass and the weight change. C. the mass remains the same, but the weight changes. D. the mass changes, but the weight remains the same.

Clicker Question A temperature of 30 degrees Celsius is equivalent to A. 303 K B. 300 K C. 243 K D. 247 K

Clicker Question 1 Mg = 1000 kg. Which of the following would be a correct conversion factor for this relationship? A. x 1000. B. x 1/1000. C. ÷ 1000. D. 1000 kg/1 Mg. 54

Clicker Question The conversion factor used to convert joules to calories changes A. the quantity of energy measured but not the numerical value of the measurement. B. neither the numerical value of the measurement nor the quantity of energy measured. C. the numerical value of the measurement but not the quantity of energy measured. D. both the numerical value of the 55 measurement and the quantity of

Clicker Question How many μg are in 0. 0134 g? – 4 A. 1. 34 x 10 B. 1. 34 x 10– 6 C. 1. 34 x 106 D. 1. 34 x 104 56

Clicker Question If 50. 0 m. L of corn syrup have a mass of 68. 7 g, the density of the corn syrup is A. 0. 737 g/m. L. B. 0. 727 g/m. L. C. 1. 36 g/m. L. D. 1. 37 g/m. L. 57

Clicker Question What is the volume of a pure gold coin that has a mass of 38. 6 g? The density of gold 3 is 19. 3 g/cm. A. 0. 500 cm 3 B. 2. 00 cm 3 C. 38. 6 cm 3 3 D. 745 cm 58

Clicker Question How many g are in 0. 0134 g? – 2 A. 1. 34 x 10 B. 1. 34 x 10– 6 C. 1. 34 x 106 D. 1. 34 x 104 59

Clicker Question Express the density 5. 6 g/cm 3 in kg/m 3. A. 5. 6 106 kg/m 3 3 3 B. 5. 6 10 kg/m C. 0. 56 kg/m 3 D. 0. 0056 kg/m 3 60