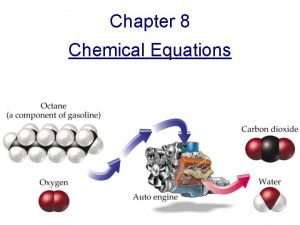
CHEMISTRY CHAPTER 9 Chemical Reactions 9 1 Reactions





































- Slides: 45

CHEMISTRY CHAPTER 9 Chemical Reactions

9. 1 Reactions and Equations Objectives: 1. Recognize evidence of chemical change 2. Represent chemical reactions with equations 3. Balance chemical equations

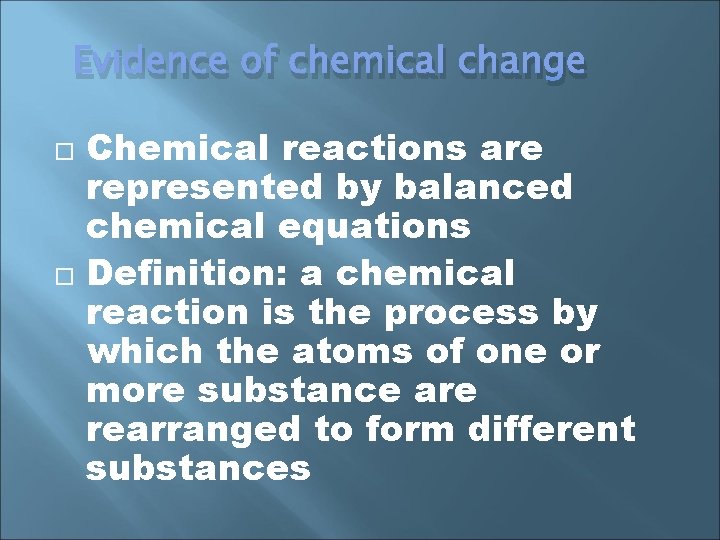
Evidence of chemical change Chemical reactions are represented by balanced chemical equations Definition: a chemical reaction is the process by which the atoms of one or more substance are rearranged to form different substances

Evidence of chemical change can be indicated by a temperature change Other examples include color change, production of a gas (usually indicated by bubbles), and the formation of a solid called a precipitate

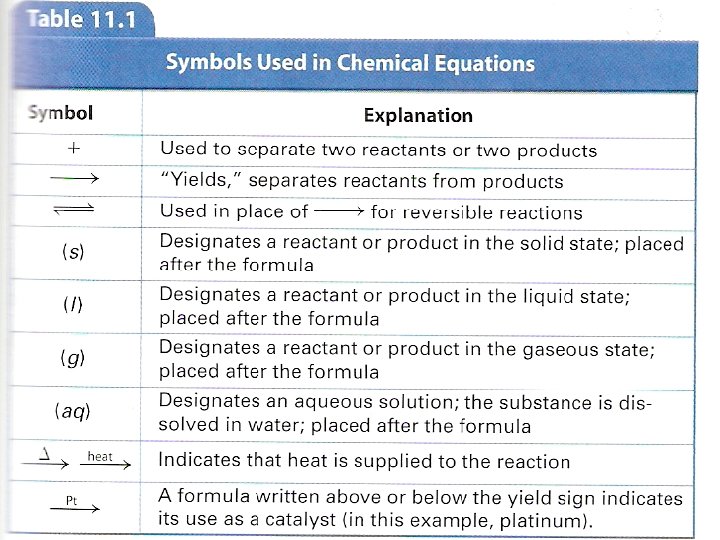
Representing Chemical Reactions with Equations Definition: reactants are the starting substances in a chemical reaction Reactants are separated from each other by plus signs on the left side of the equation Definition: products are the substances formed during the reaction

Products are separated from each other by a plus sign on the right side of the equation Reactants and products are separated from each other by an arrow The arrow means “yields”, “react to produce”, “produces”, or other similar meanings


Definition: word equations are statements indicating reactant and products of chemical reactions Ex: Aluminium + Hydrochloric acid Aluminium chloride + Hydrogen Compare the word equation to the chemical equation for the same compounds

(skeleton equation): Al + HCl Al. Cl 3 + H 2 This skeleton chemical equation is not balanced and does not give the phase: 2 Al(s) + 6 HCl(aq) 2 Al. Cl 3(s) + 3 H 2(g) (this is balanced) Definition: a skeleton equation uses chemical formulas rather than words A skeleton equation includes the phase but is not balanced

Balancing Equations Definition: a chemical equation is a statement that uses chemical formulas to show the identities and relative amounts of the substances involved in a chemical reaction Definition: a coefficient in a chemical equation is a whole number written in front of a reactant or a product

Steps for balancing 1. Write the skeleton equation 2. Count the atoms of the elements in the reactants 3. Count the atoms of the elements in the products 4. Change the coefficients to make the number of atoms of each element equal on both sides of the equation

5. WRITE THE COEFFICENTS IN THE LOWEST POSSIBLE RATIO 6. Check your work! Does it pass the laugh test? ? Finally, all chemical equations obey the law of conservation of mass The mass of all the reactants will always equal the mass of all the products in a chemical equation


9. 2 Classifying Chemical Reactions Objectives: 1. Classify chemical reactions 2. Identify the characteristics of different classes of chemical reactions

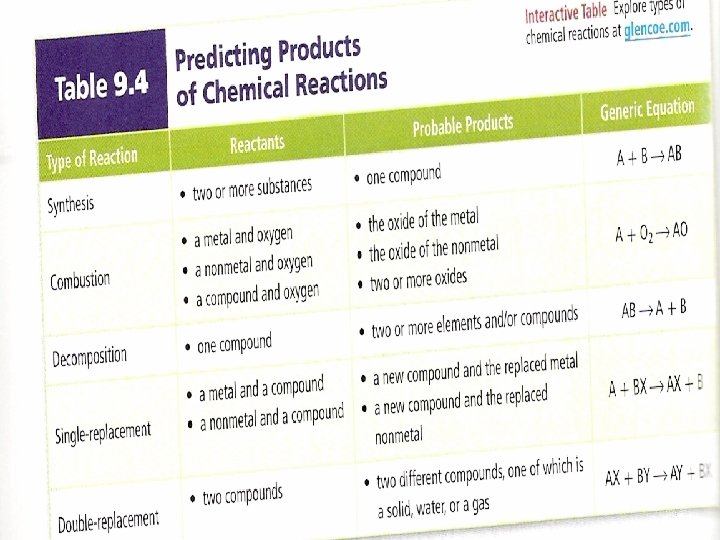
Classifying the Five Types of Reactions There are many types of chemical reactions & not all fit neatly into a pattern By learning to recognize patterns of chemical behavior, you can learn to classify chemical compounds Recognizing patterns, however, will help to learn to predict products Five types we will study include synthesis, decomposition, combustion, single replacement and double replacement

Synthesis Reactions Definition: a synthesis reaction is a chemical reaction in which two or more substances produce a single product Synthesis reactions are sometimes called combination reactions because the reactants combine with each other to produce a single product

The reactants of the most common combination reactions are either 2 elements or 2 compounds The product of a combination reaction is always a compound Fe(s) + S(s) → Fe. S(s) iron(II) sulfide OR 2 Fe(s) + 3 S(s) → Fe 2 S 3(s) iron(III) sulfide

Some nonmetal oxides react with water to produce acid: SO 2(g) + H 2 O(l) → H 2 SO 3(aq) sulfurous acid Some metallic oxides react with water to produce a base: Ca. O(s) + H 2 O(l) → Ca. OH 2(aq) calcium hydroxide When two nonmetals combine, more than one product might be possible:

S(s) + O 2(g)→ SO 2(g) sulfur dioxide 2 S(s) + 3 O 2(g)→ 2 SO 3(g) sulfur trioxide Other examples of 2 compounds combining to become 1 product: Ca. O(s) + H 2 O(l) →Ca(OH) 2(s) 2 Na + Cl 2(g) → 2 Na. Cl(s)

Decomposition Reactions Some substances break down into simpler compounds when they react These reactions may require heat or energy (from heat, light or electricity) Definition: In decomposition reactions, a single compound is broken down into two or more products In some ways a decomp reaction is the opposite of a synthesis reaction

The products can be any combination of elements and compounds It can be difficult to predict the products of decomposition reactions Rapid decomp reactions that produce gaseous products can cause explosions Ex: Ca. CO 3 → Ca. O(s) + CO 2(g) Ex: NH 4 NO 3(s) →N 2 O(g) +2 H 2 O(g)

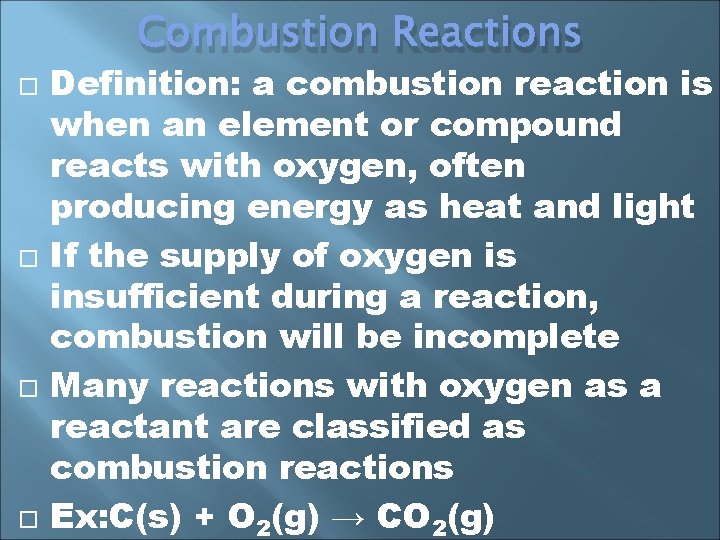
Combustion Reactions Definition: a combustion reaction is when an element or compound reacts with oxygen, often producing energy as heat and light If the supply of oxygen is insufficient during a reaction, combustion will be incomplete Many reactions with oxygen as a reactant are classified as combustion reactions Ex: C(s) + O 2(g) → CO 2(g)

We will study the hydrocarbon plus oxygen reactions Combustion reactions can be tough to balance because you have O 2 as a reactant (2 oxygens) and H 2 O as a product (1 oxygen) 2 C 2 H 18(l) + 25 O 2(g)→ 16 CO 2 + 18 H 2 O(l) The products will be carbon dioxide and water There are exceptions, but for now, look for the oxygen on the reactant side, & carbon dioxide and water on the product side for the hydrocarbon/oxygen reactions

Single Replacement Reactions Definition: in single replacement reactions, the atoms of one element replace the atoms of a second element in a compound These are also called single displacement reactions Ex: potassium replaces hydrogen (which is released as a flammable gas)-2 K(s) + 2 H 2 O(l)→ 2 KOH +H 2(g)

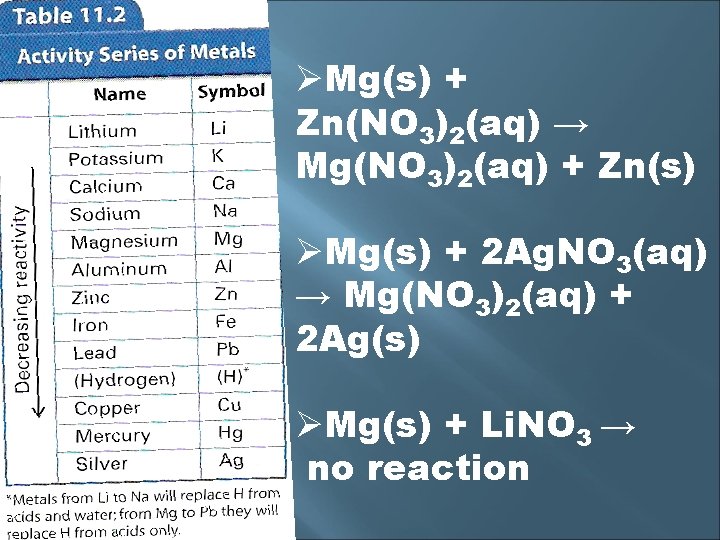
Whether one metal displaces another metal or hydrogen is determined by the activity series The activity series lists metals in order of decreasing reactivity A reactive metal will replace any metal listed below it in the activity series Look at the metallic element first & see if the element replaces the metal in the compound

ØMg(s) + Zn(NO 3)2(aq) → Mg(NO 3)2(aq) + Zn(s) ØMg(s) + 2 Ag. NO 3(aq) → Mg(NO 3)2(aq) + 2 Ag(s) ØMg(s) + Li. NO 3 → no reaction

The halogens are their own activity series in which nonmetal replaces nonmetal The reactivity is determined by the periodic table Go down the halogen column with fluorine being the most reactive and iodine the least reactive Ex: F 2(g) + 2 Na. Br(aq)→ 2 Na. F +Br 2(l) Ex: Br 2(g) + 2 Na. F(aq)→ NR

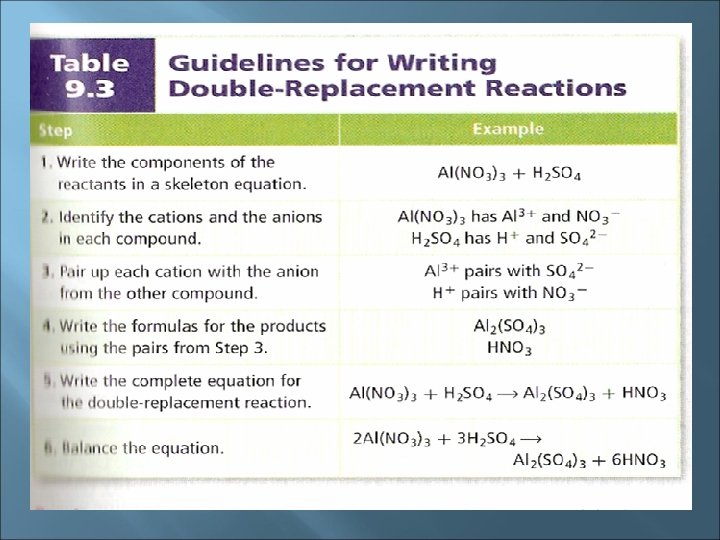
Double Replacement Reactions Double replacement (displacement) reactions involve an exchange of positive ions between two reacting compounds All double replacement reactions produce either water, a precipitate or a gas Without those products, you get NR 1. one product is slightly soluble and precipitates: Na 2 S(aq) + Cd(NO 3)2(aq) → Cd. S(s) +

Definition: a precipitate is a solid that settles out a liquid mixture Precipitate formation involves a change of state (liquid → solid) & signifies a chemical reaction 2. One product is a gas that bubbles out of the mixture: 2 Na. CN(aq) + H 2 SO 4(aq) → 2 HCN(g) + Na 2 SO 4(aq) 3. one product is a molecular compound such as water: Ca(OH)2(aq) + 2 HCl(aq) → Ca. Cl 2(aq) + 2 H 2 O(l)


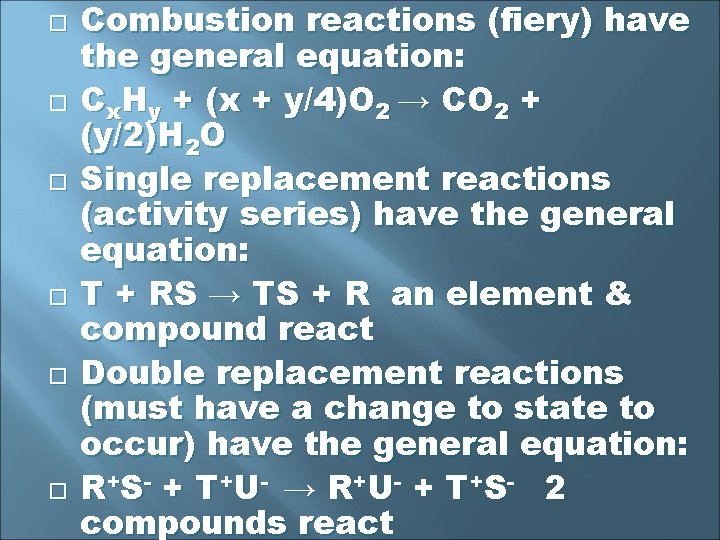
Predicting Products of a Chemical Reaction Synthesis reactions (baking a cake) have the general equation: A + B → AB 2 or more substances become 1 product Decomposition reactions (CSI stinky dead stuff) have the general equation: RS → R + S 1 substance becomes 2 or more substances

Combustion reactions (fiery) have the general equation: Cx. Hy + (x + y/4)O 2 → CO 2 + (y/2)H 2 O Single replacement reactions (activity series) have the general equation: T + RS → TS + R an element & compound react Double replacement reactions (must have a change to state to occur) have the general equation: R + S - + T + U- → R + U- + T + S - 2 compounds react


9. 3 Reactions in Aqueous Solutions Objectives: 1. Describe aqueous solutions 2. Write complete ionic and net ionic equations for chemical reactions in aqueous solutions 3. Predict whether reactions in aqueous solutions will produce a precipitate, water or a gas

Aqueous Solutions Definition: an aqueous solution contains one or more substances called solutes dissolved in water Water is the solvent Definition: a solvent is the most plentiful substance in a solution Definition: a solute is the least plentiful substance (often a solid salt) in a solution There will be no reaction in aqueous solution unless a product has undergone a phase change

Some solutes are molecular such as sucrose and ethanol These solutes exist as molecules in aqueous solutions Some molecular solutes like hydrogen chloride form ions in aqueous solution Molecular solids that form ions in aqueous solutions are acids Ionic compounds might be solutes in aqueous solutions

Complete Ionic Equations Definition: a precipitate is a solid formed from mixing 2 aqueous solutions Aqueous double-replacement reactions tend to form ionic compounds by their formula units Ag. NO 3(aq) + Na. Cl(aq)→ Ag. Cl(s) + Na. NO 3(aq) Definition: a complete ionic equation is an ionic equation that shows all the particles in a solution as they exist

Net Ionic Equations Definition: net ionic equations are ionic equations that include only those particles that participate in the reaction Definition: spectator ions are particles that do not participate in the reaction We can write the complete ionic equation to show the cations and anions: Ag+(aq) + NO 3 -(aq) + Na+(aq) + Cl(aq) → Ag. Cl(s) + Na+(aq) + NO -(aq)

We can also eliminate ions that do not directly participate in the reaction In order to be involved in the reaction, the ion must undergo a change of state (phase change) Therefore, you must include the state when writing these equations Spectators will appear on both sides of the equation as aqueous ions

Here is the same reaction with the spectator ions removed: Ag+(aq) + Cl-(aq) → Ag. Cl(s) This is the called the net ionic equation The net ionic equation shows only the particles that actually take part in the reaction You must balance the ionic charge in these equations The net ionic charge on each side of the equation must be zero Q: How are ionic equations different from chemical equations?

Look at this reaction: Pb(s) + Ag. NO 3(aq) → Ag(s) + Pb(NO 3)2(aq) The nitrate is a spectator The net ionic equation is Pb(s) + Ag+ → Ag(s) + Pb 2+(aq) ***this is unbalanced*** The atom numbers are ok, but the ionic charges are not, so coefficients are used to balance the charges: Pb(s) + 2 Ag+ → 2 Ag(s) + Pb 2+(aq) **now it is balanced** Generally single and double replacement reactions can be written as ionic equations

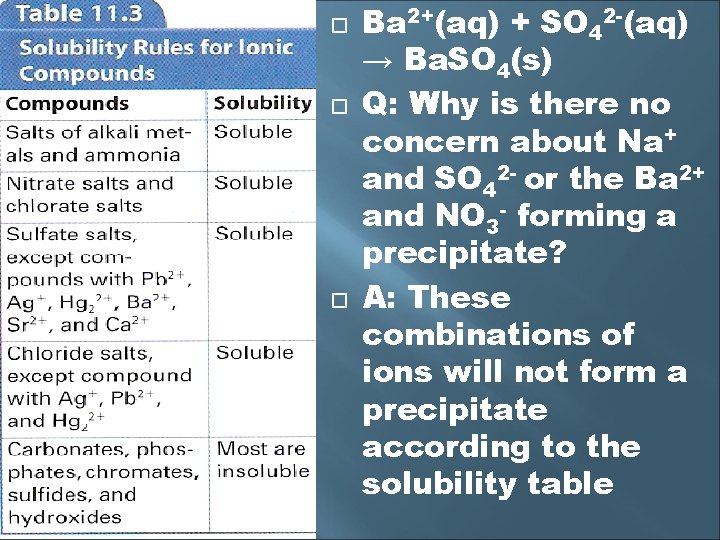
Predicting a Precipitate Use a solubility rules chart to determine precipitate formation Q: Will we get a precipitate when aqueous solutions of Ba(NO 3)2(aq) and Na 2 SO 4(aq) are mixed? The new compounds might be Na. NO 3 and Ba. SO 4 The equation looks like this: 2 Na+(aq) + SO 42 -(aq) + Ba 2+(aq) + 2 NO 3 -(aq) → Ba. SO 4(s) + 2 Na+(aq) + 2 NO 3 -(aq) the sodium and nitrate are spectators, so the net ionic equation is 2+ 2 -

Ba 2+(aq) + SO 42 -(aq) → Ba. SO 4(s) Q: Why is there no concern about Na+ and SO 42 - or the Ba 2+ and NO 3 - forming a precipitate? A: These combinations of ions will not form a precipitate according to the solubility table

Reactions that Produce Water Unlike precipitate reactions, no reaction is observable when water forms Chem rxn: HBr(aq) + Na. OH(aq) → H 2 O(l) + Na. Br(aq) Complete ionic: H+(aq) + Br-(aq) +Na+(aq) + OH-(aq) → H 2 O(l) + Na+(aq) + Br-(aq) Net ionic: H+(aq) + OH-(aq) → H 2 O(l)

Reactions that Produce a Gas Common gases produced are HCN, CO 2 & H 2 S Chem rxn: 2 HI + Li 2 S(aq) →H 2 S(g) +2 Li(aq) Complete ionic: 2 H+(aq) + 2 I(aq) + 2 Li+(aq) + S 2 -(aq) → H 2 S(g) + 2 Li+(aq) + 2 I-(aq) Net ionic: 2 H+(aq) + S 2 -(aq) → H 2 S(g)
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Synthesis reaction
Synthesis reaction What is the type of reaction
What is the type of reaction What is an active metal
What is an active metal Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds What is the chemical formula for tetranitrogen heptoxide?
What is the chemical formula for tetranitrogen heptoxide? Redox reaction example
Redox reaction example Types of reactions chemistry
Types of reactions chemistry 5 types of reactions in chemistry
5 types of reactions in chemistry Type of reactions chemistry
Type of reactions chemistry Batlum
Batlum Chemistry reactions
Chemistry reactions Stoichiometry island diagram
Stoichiometry island diagram Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Redox rules
Redox rules How to identify types of chemical reactions
How to identify types of chemical reactions Reaction type
Reaction type Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions The calculation of quantities in chemical reactions
The calculation of quantities in chemical reactions Principles of immuno chemical reactions
Principles of immuno chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products synthesis
Predicting products synthesis Activity series of metals
Activity series of metals Unit 11 chemical reactions
Unit 11 chemical reactions Lesson 68 toxic reactions chemical equations answer key
Lesson 68 toxic reactions chemical equations answer key Four types of chemical reactions
Four types of chemical reactions