Chemistry Chapter 7 8 and 9 Jeopardy Jennie























































- Slides: 65

Chemistry Chapter 7, 8, and 9 Jeopardy Jennie L. Borders

Round 1 – Chapters 7 and 8

Electron Dot Structure Vocabulary and econfiguration Ionic Compounds Lewis Dot Structures I, P, or N and Dipole Moments Intermolecular Forces 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500

Round 2 – Chapters 8 and 9 Click to go to Round 2

Vocabulary 100 What is the octet rule? Most atoms want 8 valence electrons to become stable.

Vocabulary 200 What is a formula unit? The whole number ratio of ions in an ionic compound.

Vocabulary 300 What is the electron sea model? A model that shows mobile electrons in metals.

Vocabulary 400 What is a polyatomic ion? Multiple atoms bonded together with a charge. Ex: NO 3 -

Vocabulary 500 What is a substitutional alloy and an interstitial alloy? (Include a picture. ) In a substitutional alloy, new metal atoms of a similar size replace existing metal atoms. In an interstitial alloy, new smaller metal atoms are inserted in between existing metal atoms.

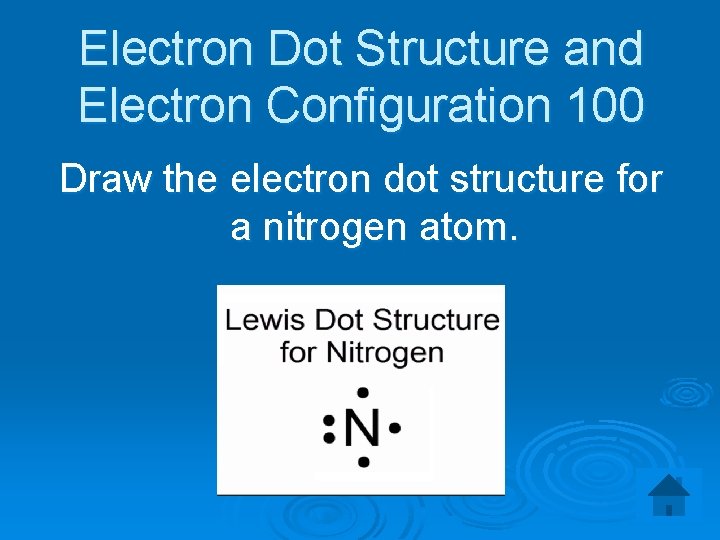
Electron Dot Structure and Electron Configuration 100 Draw the electron dot structure for a nitrogen atom.

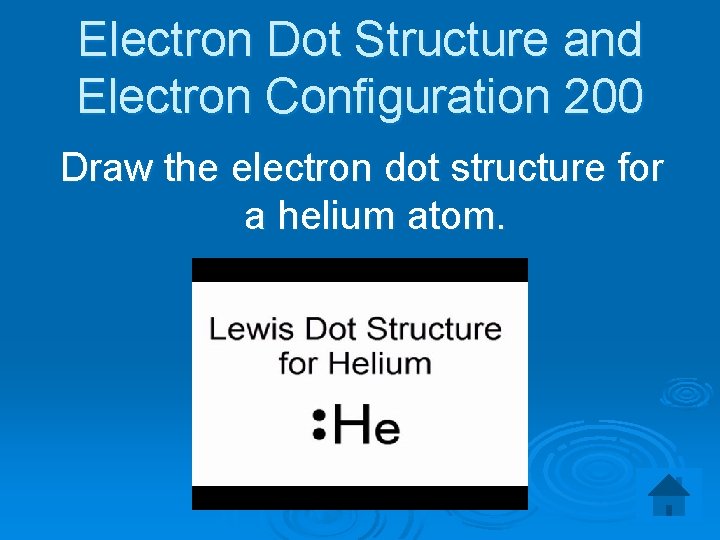
Electron Dot Structure and Electron Configuration 200 Draw the electron dot structure for a helium atom.

Electron Dot Structure and Electron Configuration 300 Write the standard electron configuration for a magnesium ion. Mg+2 = 1 s 22 p 6

Electron Dot Structure and Electron Configuration 400 Write the standard electron configuration for a phosphide ion. P-3 = 1 s 22 p 63 s 23 p 6

Electron Dot Structure and Electron Configuration 500 Write the standard electron configuration for a bromide ion. Br- = 1 s 22 p 63 s 23 p 63 d 104 s 24 p 6

Ionic Compounds 100 What is an ionic bond? Electrostatic forces between oppositely charged ions.

Ionic Compounds 200 What is the net charge on the compound sodium oxide? 0

Ionic Compounds 300 What happens to a potassium ion in order to become stable? What charge does it have? loses 1 echarge = +1

Ionic Compounds 400 Which of the following would form an ionic compound? oxygen and chlorine or barium and sulfur

Ionic Compounds 500 What are 4 characteristics of an ionic compound? metal and nonmetal cation and anion formed by ionic bonds crystalline solids high melting point conducts electricity when molten or aqueous neutral

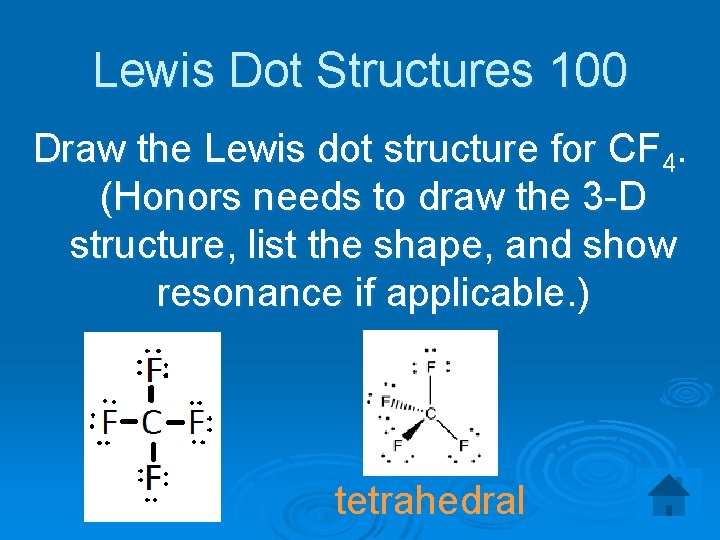
Lewis Dot Structures 100 Draw the Lewis dot structure for CF 4. (Honors needs to draw the 3 -D structure, list the shape, and show resonance if applicable. ) tetrahedral

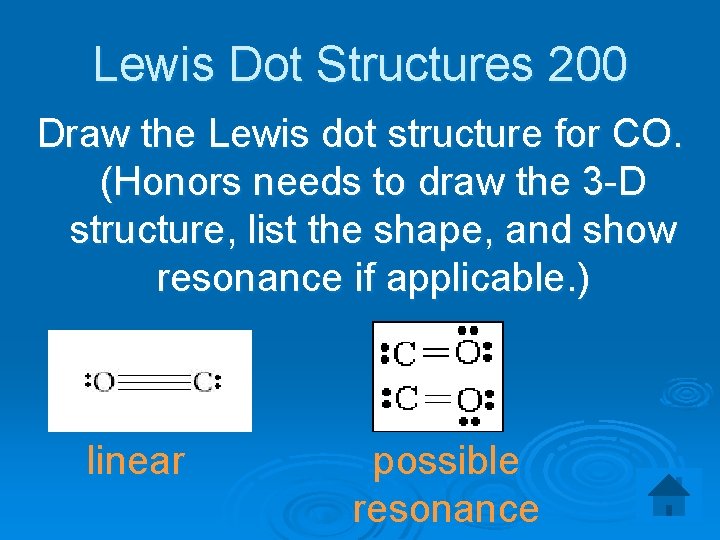
Lewis Dot Structures 200 Draw the Lewis dot structure for CO. (Honors needs to draw the 3 -D structure, list the shape, and show resonance if applicable. ) linear possible resonance

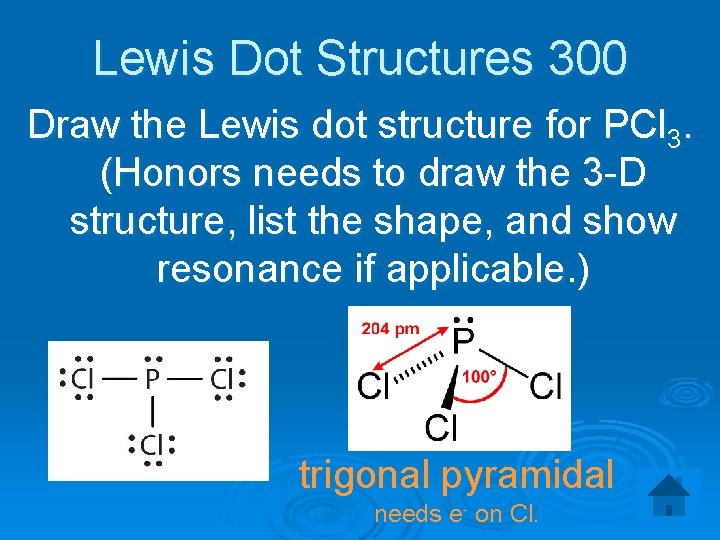
Lewis Dot Structures 300 Draw the Lewis dot structure for PCl 3. (Honors needs to draw the 3 -D structure, list the shape, and show resonance if applicable. ) trigonal pyramidal needs e- on Cl.

Lewis Dot Structures 400 Draw the Lewis dot structure for CO 3 -2. (Honors needs to draw the 3 -D structure, list the shape, and show resonance if applicable. ) trigonal planar

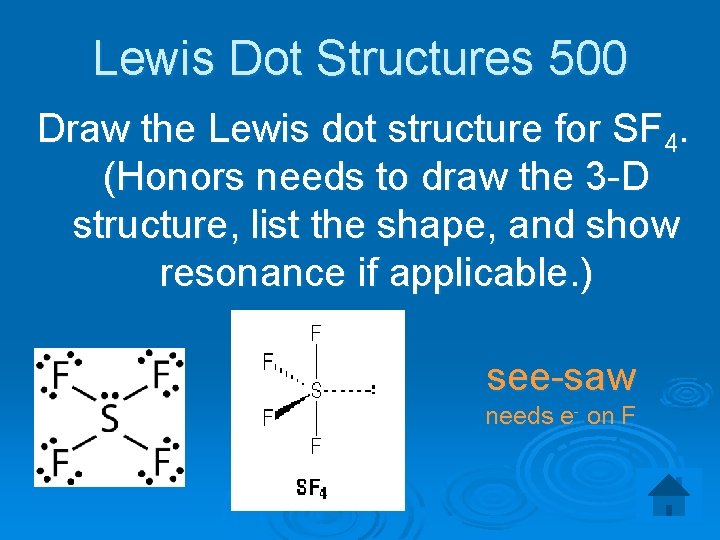
Lewis Dot Structures 500 Draw the Lewis dot structure for SF 4. (Honors needs to draw the 3 -D structure, list the shape, and show resonance if applicable. ) see-saw needs e- on F

Ionic, Polar, or Nonpolar and Dipole Moments 100 Is a Li-N bond polar, nonpolar, or ionic? ionic

Ionic, Polar, or Nonpolar and Dipole Moments 200 Is a B-H bond polar, nonpolar, or ionic? nonpolar

Ionic, Polar, or Nonpolar and Dipole Moments 300 Is an Si-O bond polar, nonpolar, or ionic? polar

Ionic, Polar, or Nonpolar and Dipole Moments 400 Draw the dipole moment for an N-N bond. N-N none

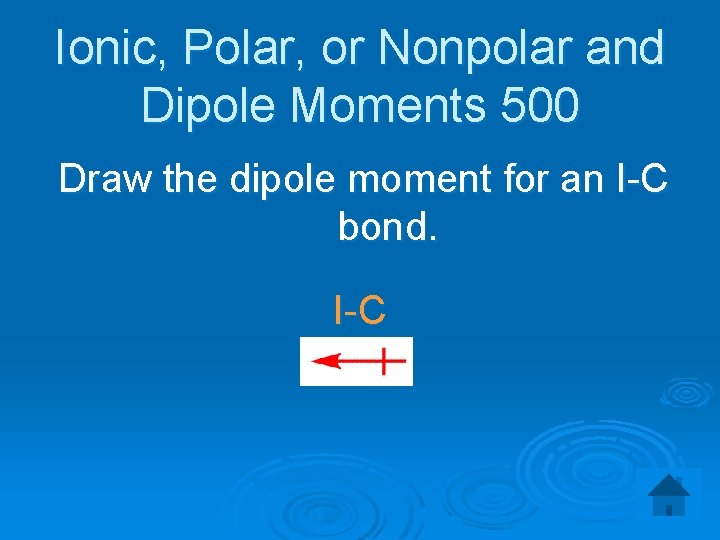
Ionic, Polar, or Nonpolar and Dipole Moments 500 Draw the dipole moment for an I-C bond. I-C

Intermolecular Forces 100 Does an H 2 molecule experience London dispersion forces, dipole attractions, or hydrogen bonding? London dispersion forces

Intermolecular Forces 200 Does an HF molecule experience London dispersion forces, dipole attractions, or hydrogen bonding? hydrogen bonding

Intermolecular Forces 300 Does a PF 5 molecule experience London dispersion forces, dipole attractions, or hydrogen bonding? dipole-dipole attractions

Intermolecular Forces 400 Does an NO 2 molecule experience London dispersion forces, dipole attractions, or hydrogen bonding? London dispersion forces

Intermolecular Forces 500 Does an Se-Br bond experience London dispersion forces, dipole attractions, or hydrogen bonding? London dispersion forces

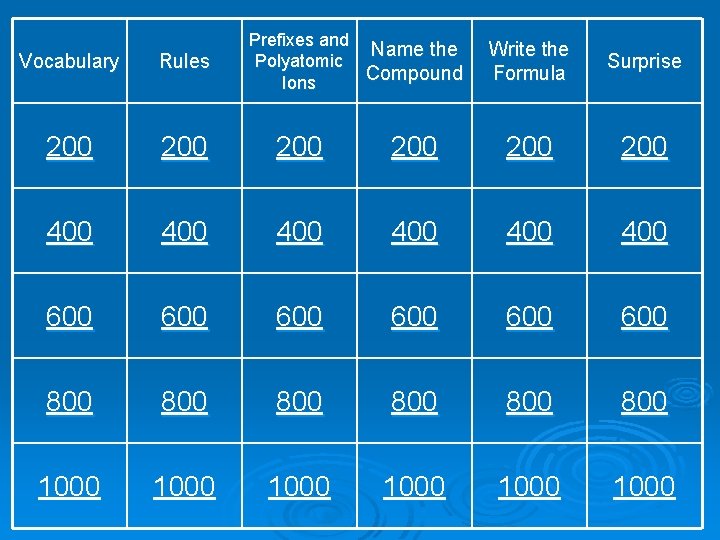
Prefixes and Name the Polyatomic Compound Ions Write the Formula Surprise 200 200 400 400 600 600 600 800 800 800 1000 1000 Vocabulary Rules 200 200 400 600

Vocabulary 200 What is the difference between a polar and a nonpolar bond? A polar bond is where electrons are shared unequally. A nonpolar bond is where electrons are shared equally.

Vocabulary 400 What is a coordinate covalent bond? A bond in which one atom donates both electrons to be shared.

Vocabulary 600 What is the difference between intermolecular forces and intramolecular forces? Intermolecular forces are between multiple molecules. Intramolecular forces are within ONE molecule.

Vocabulary 800 What is hydrogen bonding? (Be specific!) A very strong dipole-dipole attraction between the hydrogen of one molecule and an extremely electronegative element (N, O, or F) of another molecule.

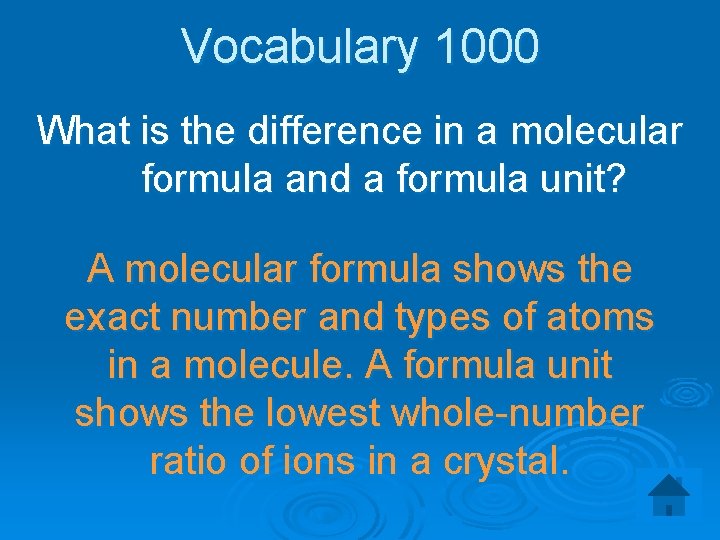
Vocabulary 1000 What is the difference in a molecular formula and a formula unit? A molecular formula shows the exact number and types of atoms in a molecule. A formula unit shows the lowest whole-number ratio of ions in a crystal.

Rules 200 When do you add prefixes to a name? When the compound only contains nonmetals.

Rules 400 What type of elements need roman numerals in the name? transition metals

Rules 600 When an acid contains oxygen and the anion ends in –ate, what do you change the ending to? -ic

Rules 800 When an acid contains oxygen and the anion ends in –ite, what do you change the ending to? -ous

Rules 1000 When an acid does not contain oxygen and the anion ends in -ide, what do you add to the name? (no partial credit) hydro- and -ic

Prefixes and Polyatomic Ions 200 What is the prefix for 4? tetra-

Prefixes and Polyatomic Ions 400 What is the prefix for 7? hepta-

Prefixes and Polyatomic Ions 600 What is the name for Cl. O 4 -? perchlorate

Prefixes and Polyatomic Ions 800 What is the name for NO 2 -? nitrite

Prefixes and Polyatomic Ions 1000 What is the formula (and charge) for oxalate? C 2 O 4 -2

Name the Compound 200 Name the following compound: Ca. CO 3 calcium carbonate

Name the Compound 400 Name the following compound: Si. Cl 4 silicon tetrachloride

Name the Compound 600 Name the following compound: Sn. Cr 2 O 7 tin (II) dichromate

Name the Compound 800 Name the following compound: Pb. Cr. O 4 lead (II) chromate

Name the Compound 1000 Name the following compound: HNO 3 nitric acid

Write the Formula 200 Write the formula for the following compound: potassium permanganate KMn. O 4

Write the Formula 400 Write the formula for the following compound: trisilicon tetranitride Si 3 N 4

Write the Formula 600 Write the formula for the following compound: copper (II) nitrite Cu(NO 2)2

Write the Formula 800 Write the formula for the following compound: carbon tetrabromide CBr 4

Write the Formula 1000 Write the formula for the following compound: acetic acid HC 2 H 3 O 2

Surprise 200 What electrons are involved in bonding? valence electrons

Surprise 400 Would Ba. Br 2 be a molecular or ionic compound? ionic

Surprise 600 List the intermolecular forces in order of weakest to strongest. London < Dipole < Hydrogen dispersion dipole bonding

Surprise 800 What element is normally first in the formula for an acid? hydrogen

Surprise 1000 Which bond is the most polar? H-F, H-C, H-H, or H-N H-F
 Jennie borders chemistry
Jennie borders chemistry Jennie borders chemistry
Jennie borders chemistry Jennie turner
Jennie turner Jennie finch pitching video slow motion
Jennie finch pitching video slow motion Jennie reid elementary
Jennie reid elementary Jennie ellis australia immigration
Jennie ellis australia immigration Jennie reeves radiographers agency
Jennie reeves radiographers agency Marvin gayes father
Marvin gayes father Algae biodiesel
Algae biodiesel Jennie watson-lamprey
Jennie watson-lamprey Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry The periodic table jishka
The periodic table jishka Lets play jeopardy
Lets play jeopardy Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 assessment the mole answer key
Chapter 10 assessment the mole answer key Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chapter 10 study guide the mole
Chapter 10 study guide the mole Chemistry matter and change chapter 2 answer key
Chemistry matter and change chapter 2 answer key Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity Chapter 2 basic chemistry anatomy and physiology
Chapter 2 basic chemistry anatomy and physiology Chemistry chapter 7 ionic and metallic bonding
Chemistry chapter 7 ionic and metallic bonding Approach chemistry chalk chapter
Approach chemistry chalk chapter Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Importance of organic compounds
Importance of organic compounds Chapter 9 review stoichiometry
Chapter 9 review stoichiometry Love formula in chemistry
Love formula in chemistry Modern chemistry chapter 15 review answers
Modern chemistry chapter 15 review answers Chapter 14 review acids and bases
Chapter 14 review acids and bases Chapter 13 review ions in aqueous solutions
Chapter 13 review ions in aqueous solutions Chapter 12 solutions section 1
Chapter 12 solutions section 1 Law of combining volumes
Law of combining volumes Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test What functional group is ch3
What functional group is ch3 Chapter 24 nuclear chemistry answer key
Chapter 24 nuclear chemistry answer key Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers What's the difference between solution and suspension
What's the difference between solution and suspension Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Reaction of grignard reagent with acid chloride
Reaction of grignard reagent with acid chloride Modern chemistry chapter 4
Modern chemistry chapter 4 Chemistry central science 14th edition
Chemistry central science 14th edition Chapter 11 stoichiometry assessment answer key
Chapter 11 stoichiometry assessment answer key Chapter 1 introduction to chemistry
Chapter 1 introduction to chemistry Chapter 13 solutions chemistry
Chapter 13 solutions chemistry Chapter 9 review stoichiometry
Chapter 9 review stoichiometry Ap chemistry chapter 11
Ap chemistry chapter 11 Analytical chemistry chapter 1
Analytical chemistry chapter 1 Introduction to chemistry chapter 1
Introduction to chemistry chapter 1 Chemistry chapter 3 scientific measurement
Chemistry chapter 3 scientific measurement Importance of inorganic chemistry in pharmacy
Importance of inorganic chemistry in pharmacy Ap chemistry chapter 7 periodic properties of the elements
Ap chemistry chapter 7 periodic properties of the elements Organic chemistry chapter 9
Organic chemistry chapter 9 Chemistry 101 chapter 1
Chemistry 101 chapter 1 Ap chemistry chapter 8
Ap chemistry chapter 8 Chapter 6 section 1 atoms elements and compounds answer key
Chapter 6 section 1 atoms elements and compounds answer key Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 3 organic chemistry
Chapter 3 organic chemistry Chapter 2 the chemistry of life section 2-3 answer key
Chapter 2 the chemistry of life section 2-3 answer key Chapter 6 ap chemistry
Chapter 6 ap chemistry