Chemistry Chapter 6 The Periodic Law Mendeleevs Periodic







- Slides: 42

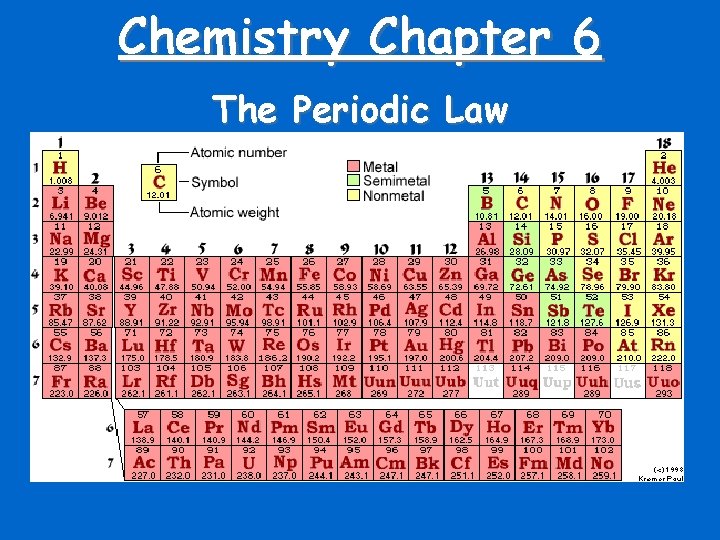
Chemistry Chapter 6 The Periodic Law


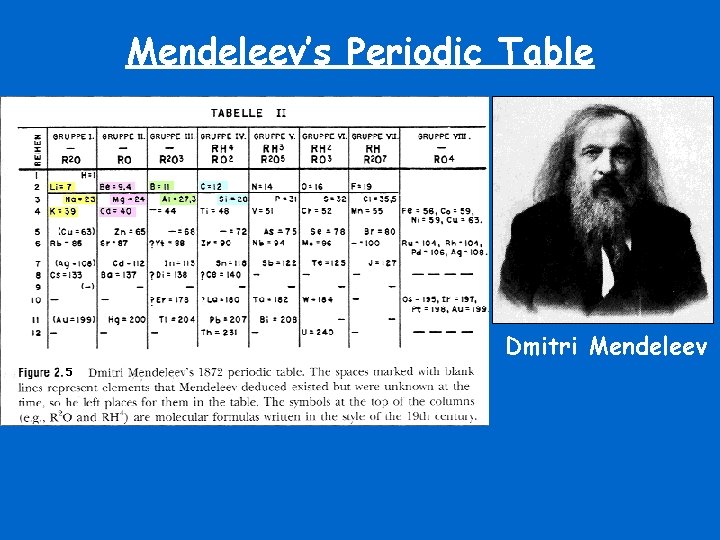
Mendeleev’s Periodic Table Dmitri Mendeleev

A Spiral Periodic Table Triangular Periodic Table “Mayan” Periodic Table

Chinese Periodic Table Stowe Periodic Table Giguere Periodic Table

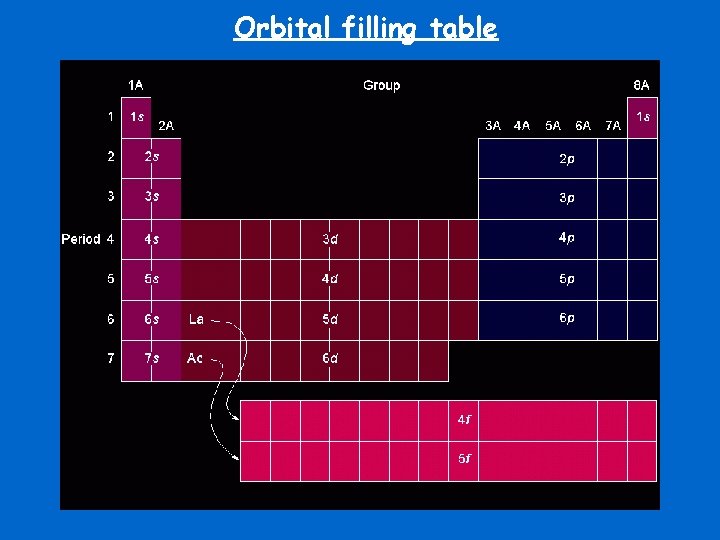
Orbital filling table

Periodic Law • Chemical properties are functions of an elements atomic number

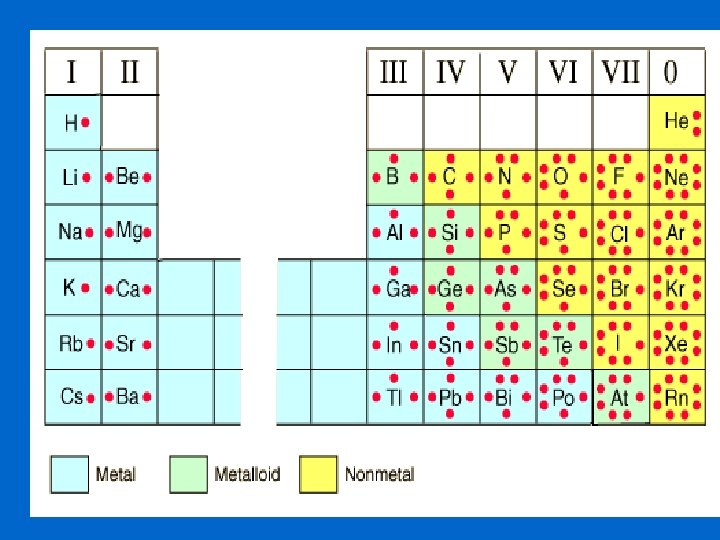
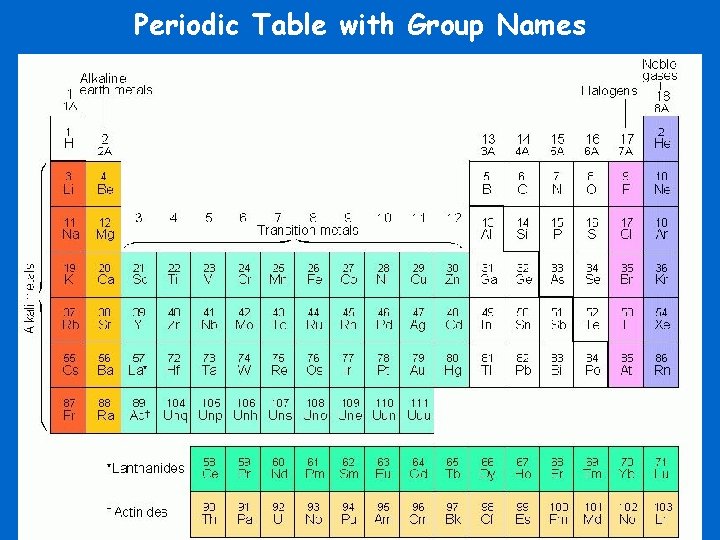
Periodic Table with Group Names

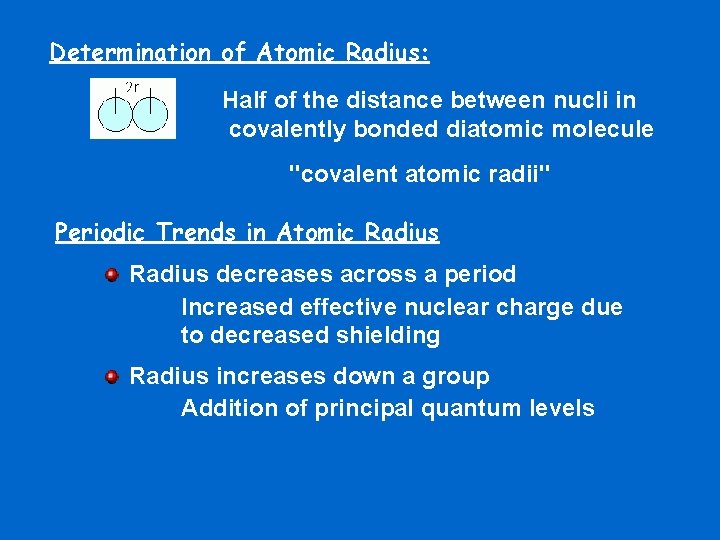
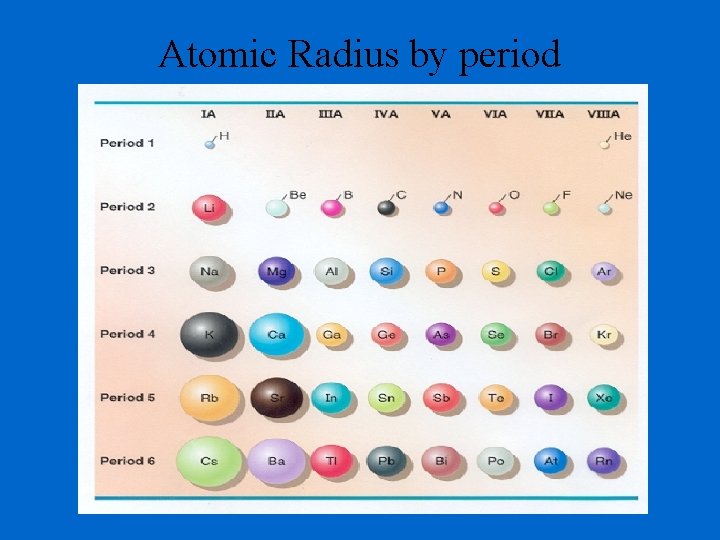
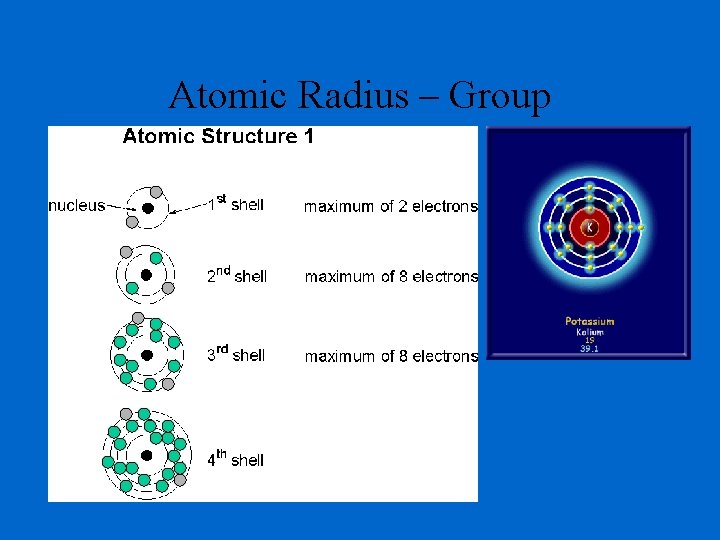
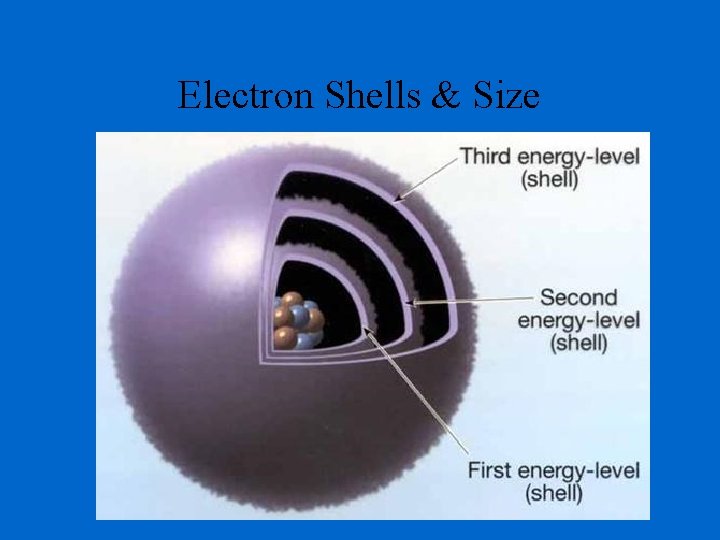
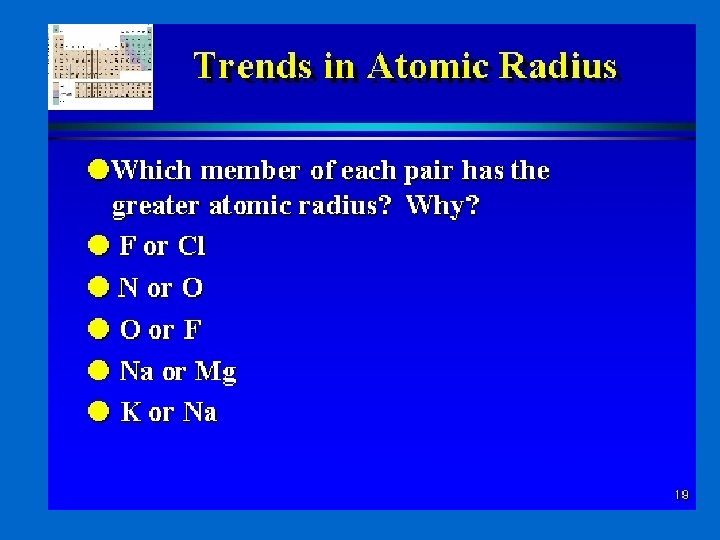
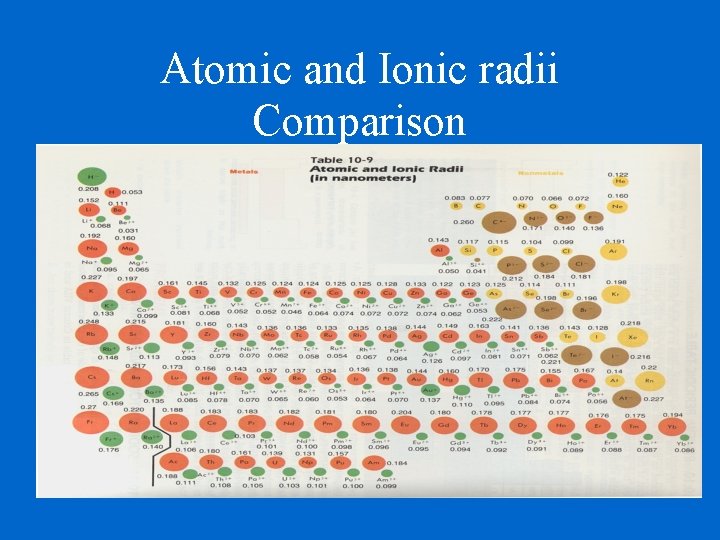
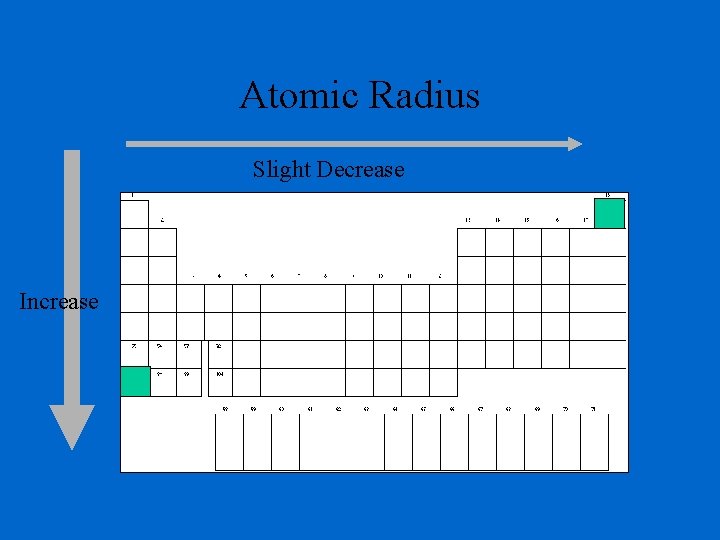
Determination of Atomic Radius: Half of the distance between nucli in covalently bonded diatomic molecule "covalent atomic radii" Periodic Trends in Atomic Radius decreases across a period Increased effective nuclear charge due to decreased shielding Radius increases down a group Addition of principal quantum levels

Atomic Radius by period

Atomic Radius – Group He Be Mg Ca

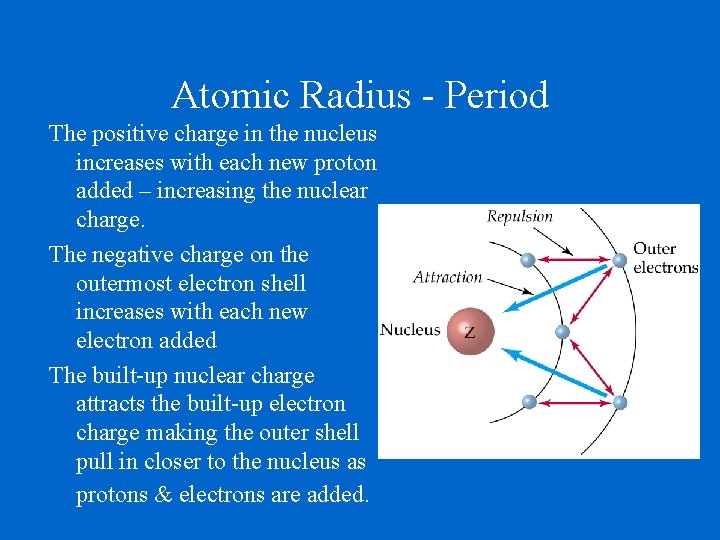
Atomic Radius - Period The positive charge in the nucleus increases with each new proton added – increasing the nuclear charge. The negative charge on the outermost electron shell increases with each new electron added The built-up nuclear charge attracts the built-up electron charge making the outer shell pull in closer to the nucleus as protons & electrons are added.

Electron Shells & Size

Check your knowledge

Ions During chemical bonding atoms become stable by forming a noble gas electron configuration. Atoms will gain or lose electrons during bonding to achieve a noble gas electron configuration. If an atom accepts or donates (gains or loses) electrons the atom will become an ion. An ion is a charged particle. Positive ions are called cations. Negative ions are called anions.

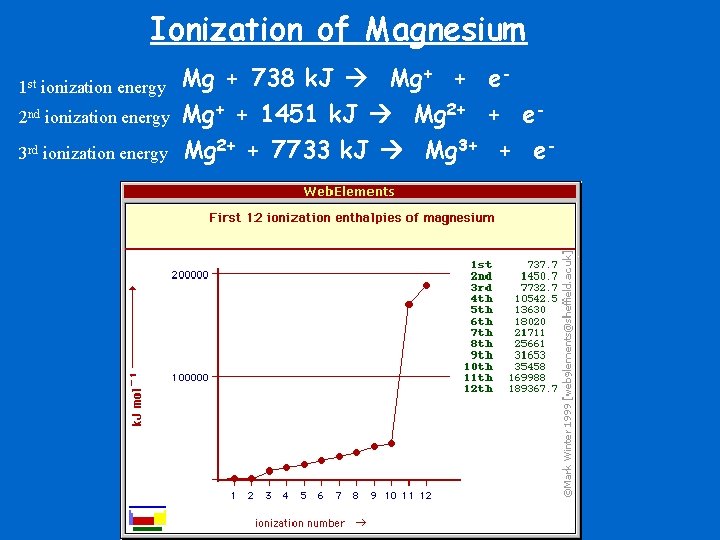
Ionization of Magnesium 2 nd ionization energy Mg + 738 k. J Mg+ + e. Mg+ + 1451 k. J Mg 2+ + e- 3 rd ionization energy Mg 2+ + 7733 k. J Mg 3+ + e- 1 st ionization energy

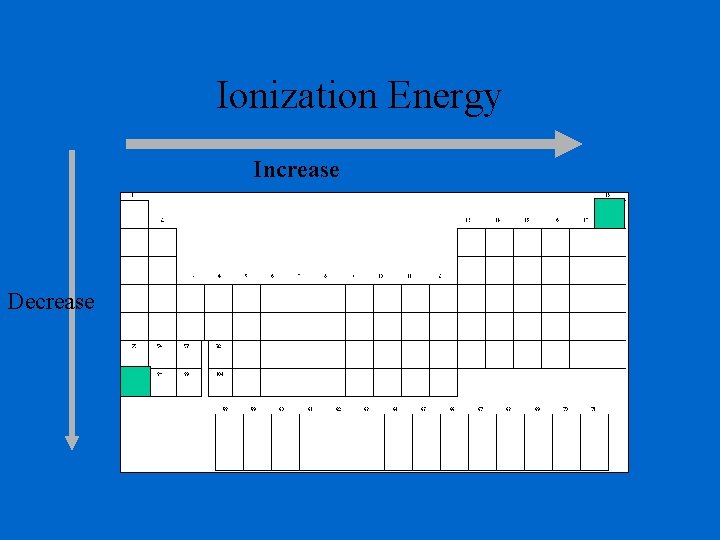
Ionization Energy • Ionization Energy is the measure of energy in KJ/mole that are required for removing an electron. • The first ionization energy is the energy needed to remove one electron from the outermost shell. • The second ionization energy is the energy needed to remove a 2 nd electron from the outermost shell after the 1 st one has been removed.

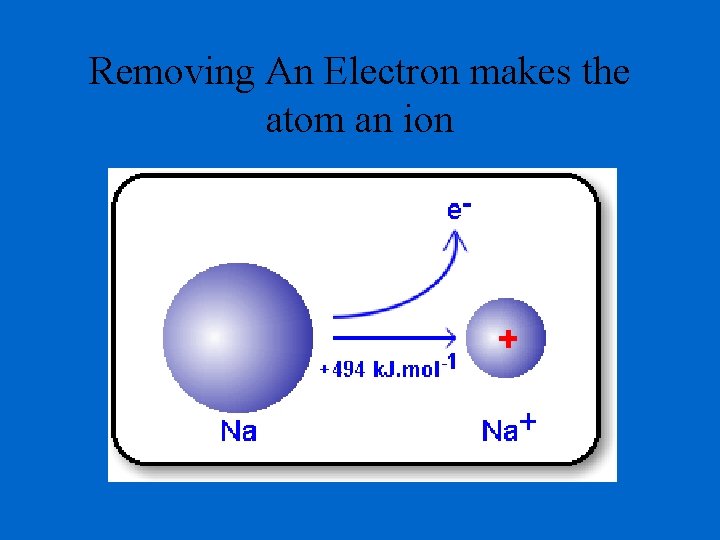
Removing An Electron makes the atom an ion

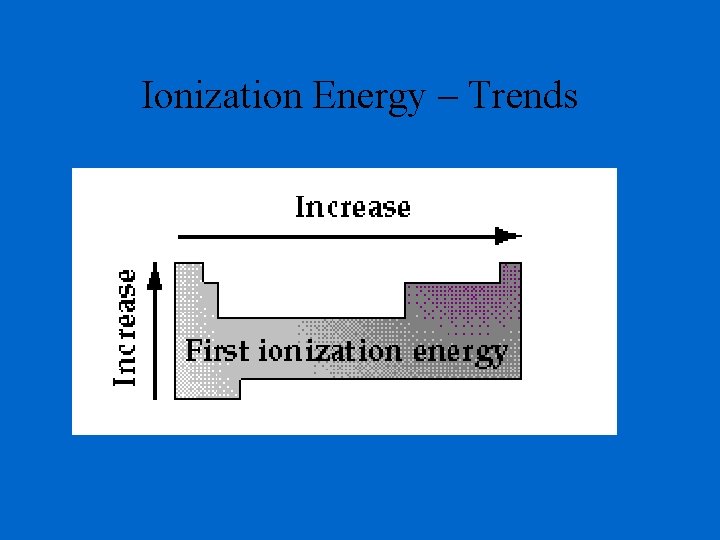
Ionization Energy – Trends

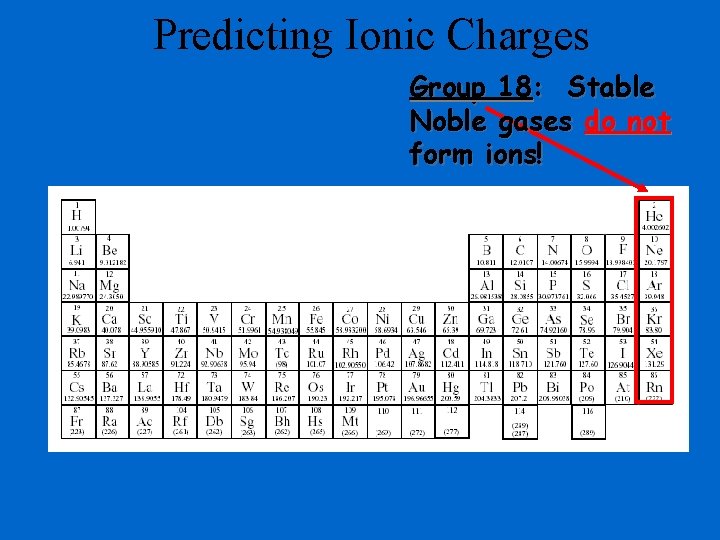
Predicting Ionic Charges Group 18: Stable Noble gases do not form ions!

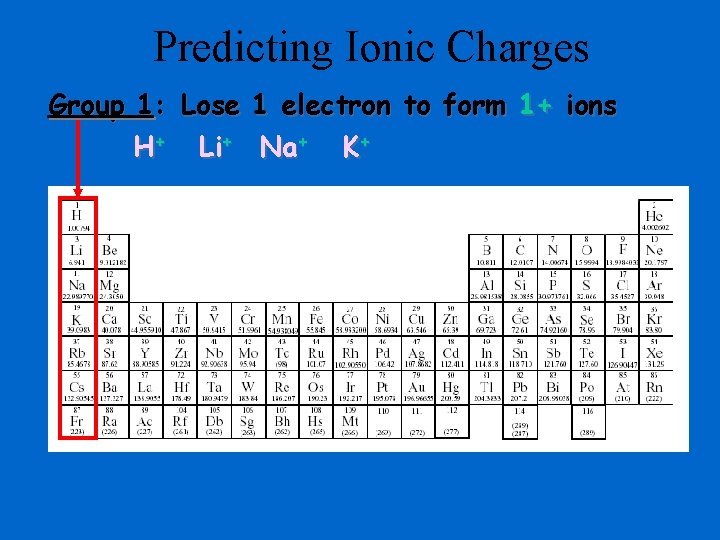
Predicting Ionic Charges Group 1: Lose 1 electron to form 1+ ions H+ Li+ Na+ K+

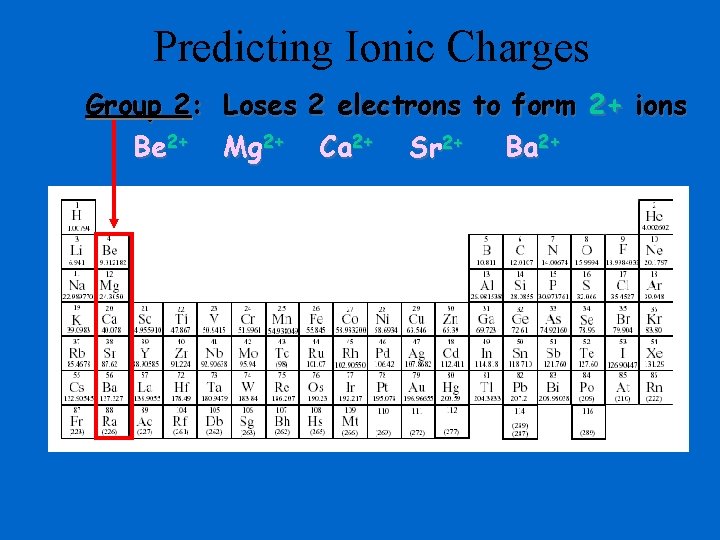
Predicting Ionic Charges Group 2: Loses 2 electrons to form 2+ ions Be 2+ Mg 2+ Ca 2+ Sr 2+ Ba 2+

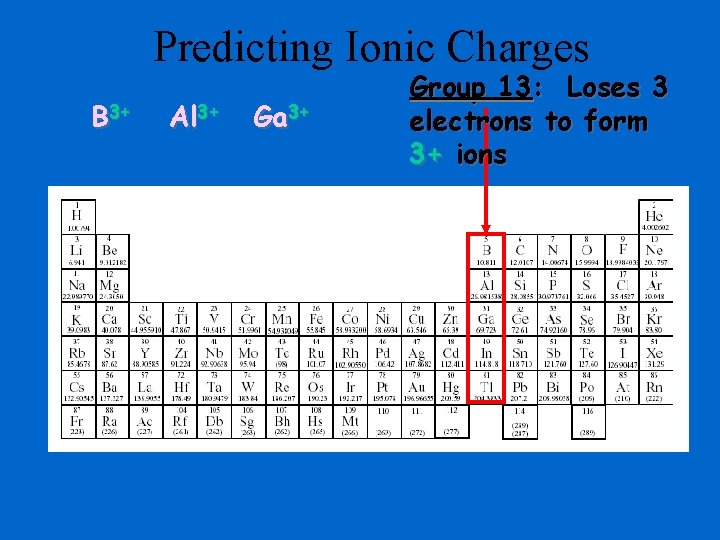
Predicting Ionic Charges B 3+ Al 3+ Ga 3+ Group 13: Loses 3 electrons to form 3+ ions

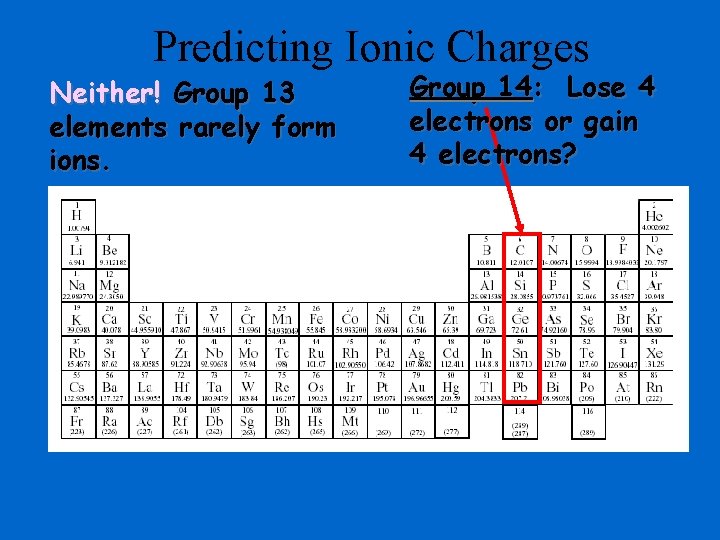
Predicting Ionic Charges Neither! Group 13 elements rarely form ions. Group 14: Lose 4 electrons or gain 4 electrons?

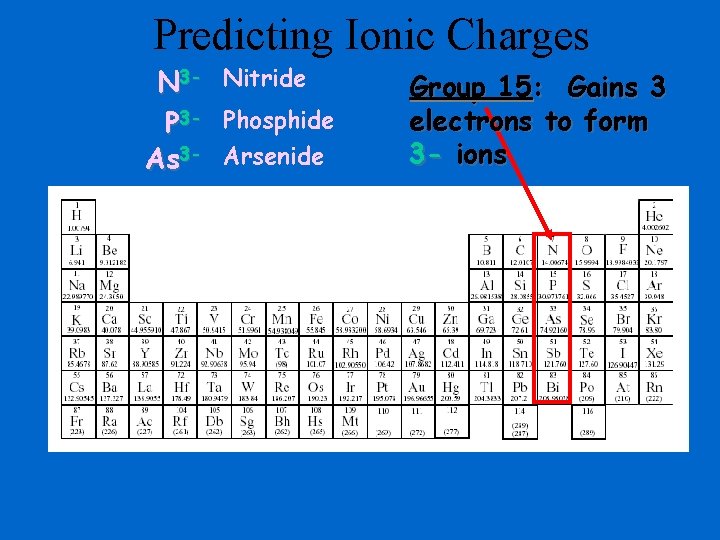
Predicting Ionic Charges N 3 - Nitride P 3 - Phosphide As 3 - Arsenide Group 15: Gains 3 electrons to form 3 - ions

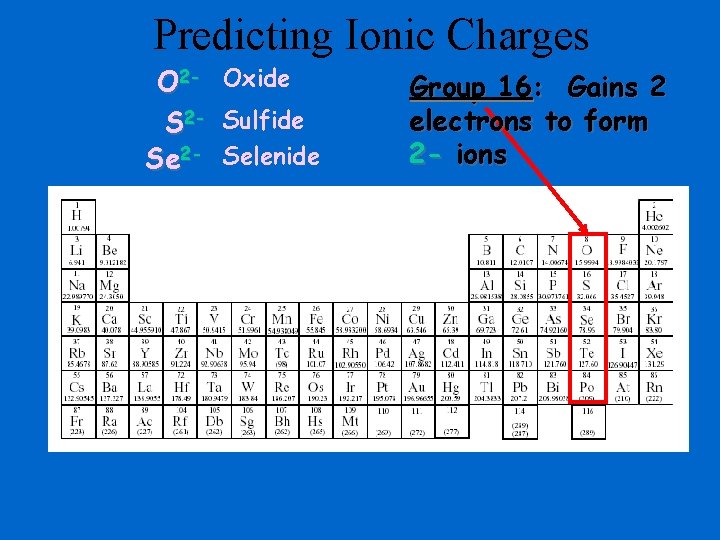
Predicting Ionic Charges O 2 - Oxide S 2 - Sulfide Se 2 - Selenide Group 16: Gains 2 electrons to form 2 - ions

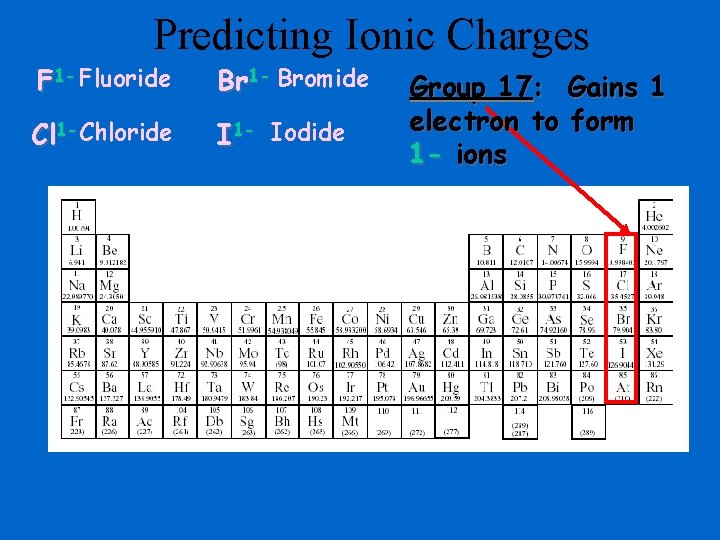
Predicting Ionic Charges F 1 - Fluoride Br 1 - Bromide Cl 1 -Chloride I 1 - Iodide Group 17: Gains 1 electron to form 1 - ions

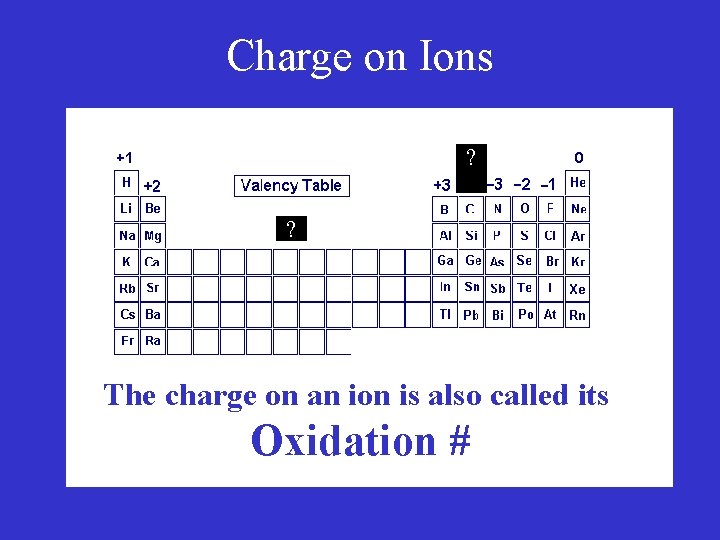
Charge on Ions ? ? The charge on an ion is also called its Oxidation #

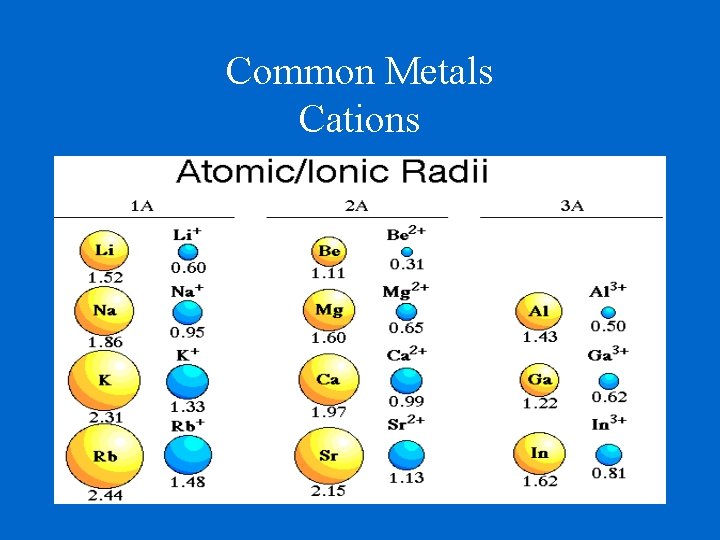
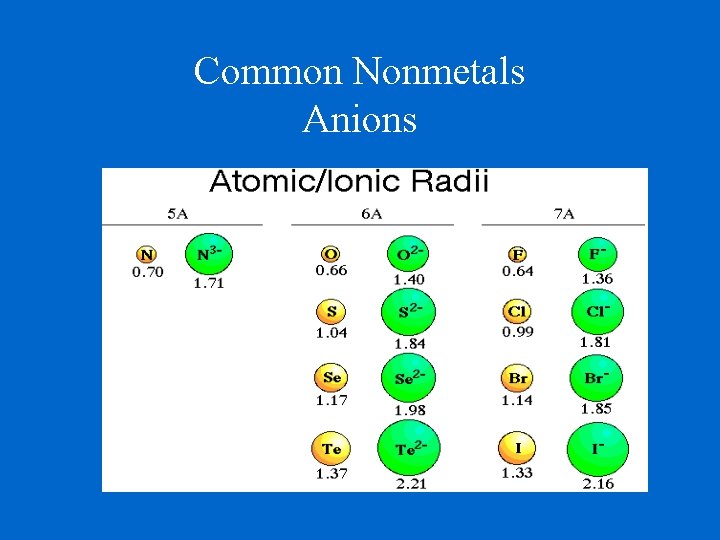
Ionic Radii Cations Positively charged ions Cations are smaller than their parent atoms because they lose electron(s) and also lose their outer energy level. Anions Negatively charged ions Anions are larger than their parent atoms because they keep their outer energy level and they cannot hold their gained electron(s) as tightly as their natural electrons

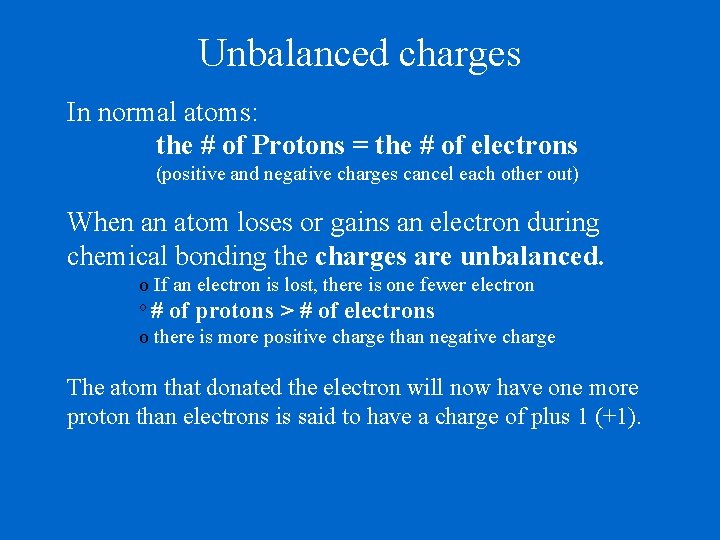
Unbalanced charges In normal atoms: the # of Protons = the # of electrons (positive and negative charges cancel each other out) When an atom loses or gains an electron during chemical bonding the charges are unbalanced. o If an electron is lost, there is one fewer electron o # of protons > # of electrons o there is more positive charge than negative charge The atom that donated the electron will now have one more proton than electrons is said to have a charge of plus 1 (+1).

Common Metals Cations

Common Nonmetals Anions

Atomic and Ionic radii Comparison

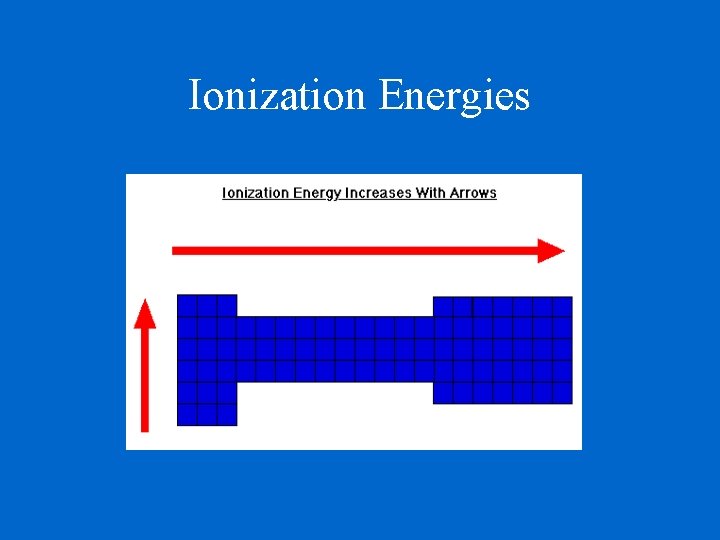
Ionization Energy - the energy required to remove an electron from an atom Increases for successive electrons taken from the same atom Tends to increase across a period Electrons in the same quantum level do not shield as effectively as electrons in inner levels Irregularities at half filled and filled sublevels due to extra repulsion of electrons paired in orbitals, making them easier to remove Tends to decrease down a group Outer electrons are farther from the nucleus

Ionization Energies

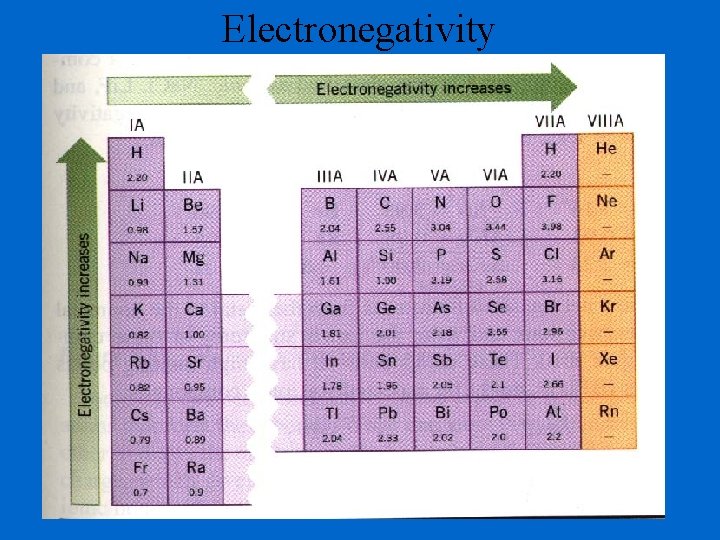
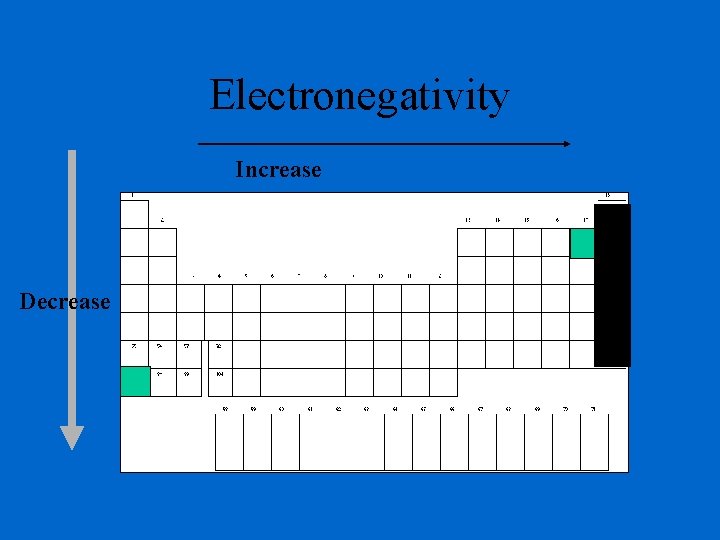
Electronegativity A measure of the ability of an atom in a chemical compound to attract electrons Electronegativities tend to increase across a period Electronegativities tend to decrease down a group or remain the same Think Greedy!!!!!

Electronegativity is the ability of an atom to attract an electron • Shielding decreases the ability to attract an electron. • Cations do not want to attract electrons because they want, instead, to lose electrons (the opposite thing). Therefore cations have a low ability to attract an electron. • Anions easily attract electrons because they want to gain electrons, making them high in electronegativity. • Noble gases, with their filled sublevels, do not want to attract electrons so they have no ability to attract electrons and therefore have no electronegativity.

Electronegativity

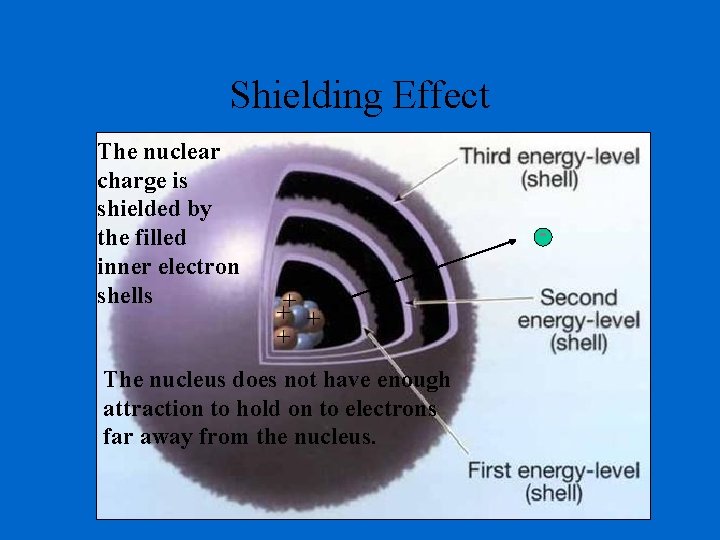
Shielding Effect The nuclear charge is shielded by the filled inner electron shells ++ + + The nucleus does not have enough attraction to hold on to electrons far away from the nucleus.

Atomic Radius Slight Decrease Increase

Ionization Energy Increase Decrease

Electronegativity Increase Decrease