Chemistry CHAPTER 6 3 IONIC BONDING What is

Chemistry CHAPTER 6. 3 IONIC BONDING
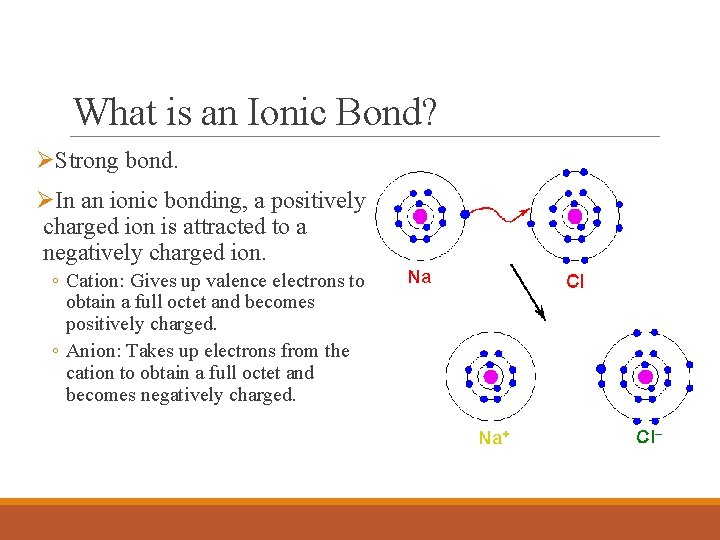
What is an Ionic Bond? ØStrong bond. ØIn an ionic bonding, a positively charged ion is attracted to a negatively charged ion. ◦ Cation: Gives up valence electrons to obtain a full octet and becomes positively charged. ◦ Anion: Takes up electrons from the cation to obtain a full octet and becomes negatively charged.

Examples Classify each compound as covalent or ionic. 1. Carbon dioxide (CO 2) 2. Potassium iodide 3. Nitrogen monoxide 4. Silver chloride. 5. Silicon dioxide 6. Sulfur dioxide. 7. Tetraphosphorous trisulfide. 8. Sodium nitride 9. Carbon monoxide

Characteristics of Ionic Bonding In an ionic crystal, ions minimize their potential energy by combining in an orderly arrangement known as crystal lattice.
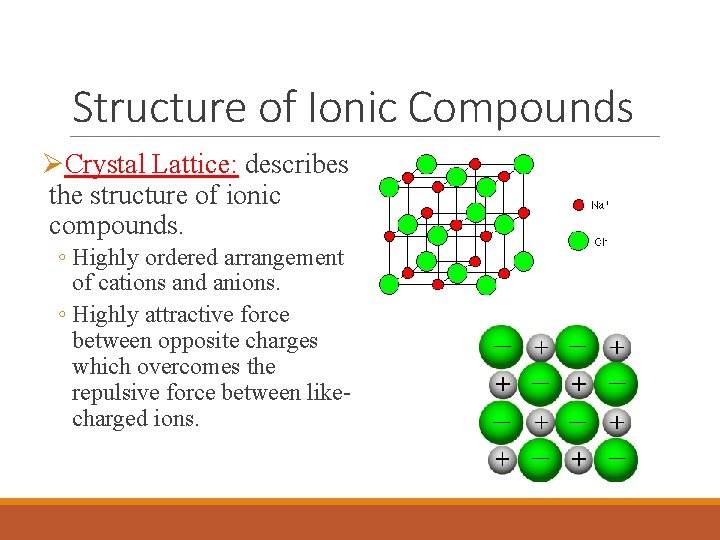
Structure of Ionic Compounds ØCrystal Lattice: describes the structure of ionic compounds. ◦ Highly ordered arrangement of cations and anions. ◦ Highly attractive force between opposite charges which overcomes the repulsive force between likecharged ions.

What is a formula unit? ØThe chemical formula of an ionic compound! ØA formula unit is the simplest collection of atoms from which an ionic compound’s formula can be established.
Why do ionic compounds conduct electricity only when they are in liquid state or dissolved in solution?
Lattice Energy ØThe amount of energy released when 1 mol of an ionic crystalline compound is formed from gaseous ions. ◦ Is a measure of Energy stored in the ionic bond AND ◦ How much energy needed to dissolve a compound in water.
Show formation of ionic bond using Lewis dot diagram: §Between sodium and chlorine

Show formation of ionic bond using Lewis dot diagram: Between magnesium and oxygen

Show formation of ionic bond using Lewis dot diagram: Between aluminum and oxygen
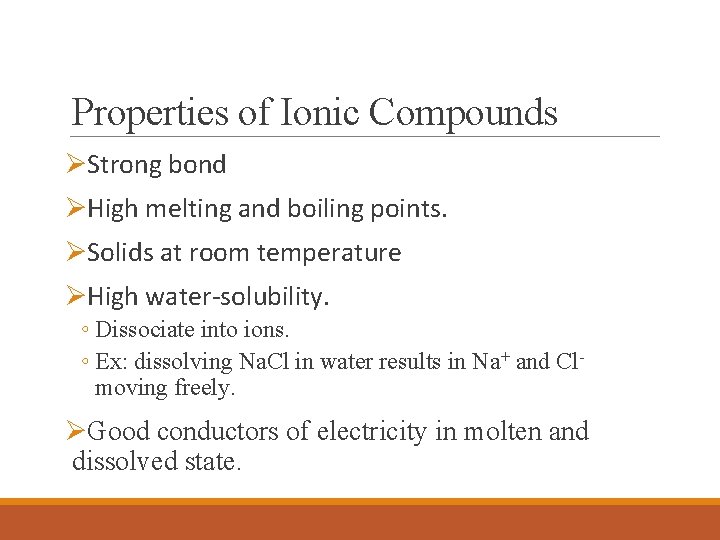
Properties of Ionic Compounds ØStrong bond ØHigh melting and boiling points. ØSolids at room temperature ØHigh water-solubility. ◦ Dissociate into ions. ◦ Ex: dissolving Na. Cl in water results in Na+ and Clmoving freely. ØGood conductors of electricity in molten and dissolved state.
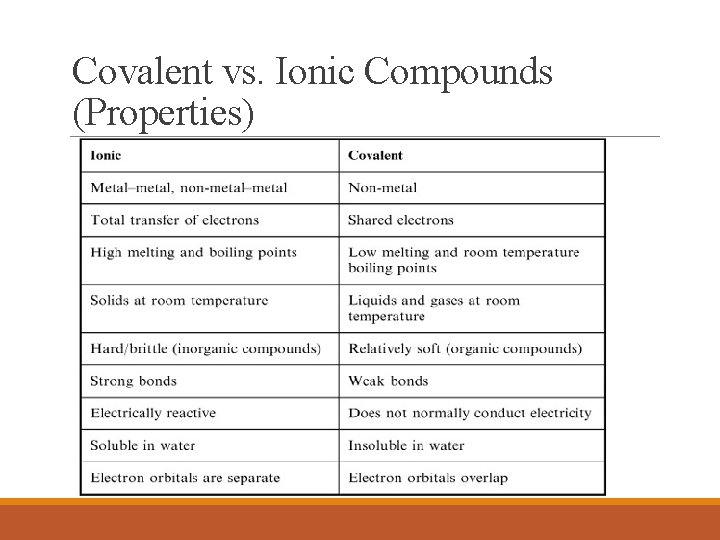
Covalent vs. Ionic Compounds (Properties)

Polyatomic Ions ØA charged group of covalently bonded atoms. ◦ Charge results from an excess of electrons—negatively charge; or a shortage of electrons—positive charge.
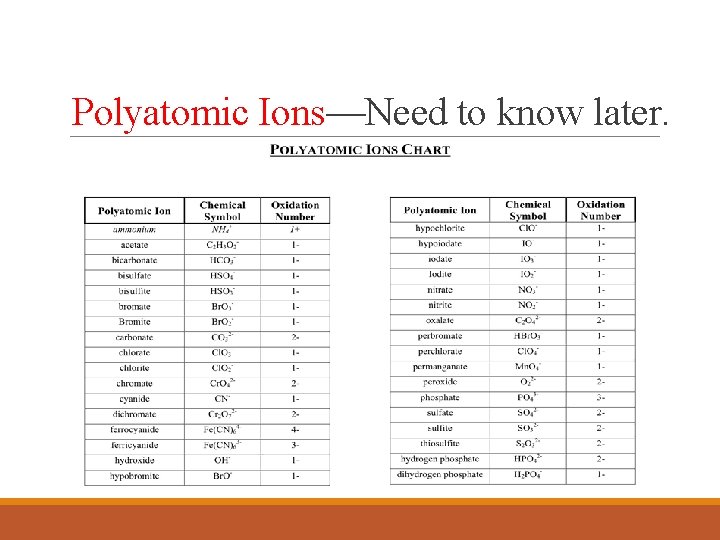
Polyatomic Ions—Need to know later.
- Slides: 15