Chemistry Chapter 3 Properties and Classification of Matter










- Slides: 26

Chemistry Chapter 3 Properties and Classification of Matter Chemistry-Matter and Change Glencoe Last revision Fall 2007

Matter – anything that has mass and volume. Mass – the amount of material that makes an object Volume – the amount of space an object takes up Your textbooks? Light from a lighthouse? The pen/pencil you are writing with? Your thoughts? Heat from a fire?

Properties of Matter Physical Property - a quality or condition of matter that can be observed or measured without changing the arrangement of atoms that make it These properties can usually be observed using our senses or measured with equipment in the lab. Chemical Properties – property that can only be observed when the arrangement of particles that make the matter are altered These properties usually tell you how a substance will react in the presence of a second substance.

Changes in Matter Physical Changes – changes in matter that do not alter the arrangement of atoms that make the matter Changes in size, shape, and STATE OF MATTER. Chemical Changes - changes in matter that DO alter the arrangement of atoms that make the matter Because you can’t see the particles to determine if arrangement has changes, you can look for clues that tell you a chemical change has occurred. Clues of a Chemical Change: 1. color change 2. production of a solid 3. production of a gas 4. release of heat, light, or sparks

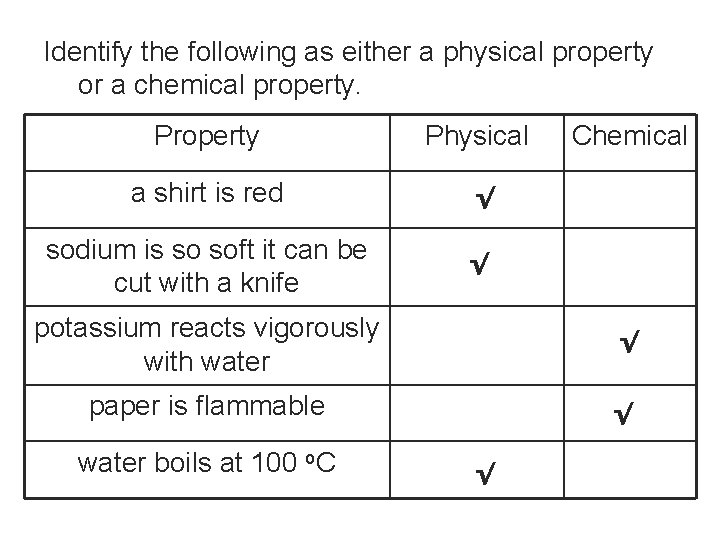
Identify the following as either a physical property or a chemical property. Property Physical a shirt is red √ sodium is so soft it can be cut with a knife √ Chemical potassium reacts vigorously with water √ paper is flammable √ water boils at 100 o. C √

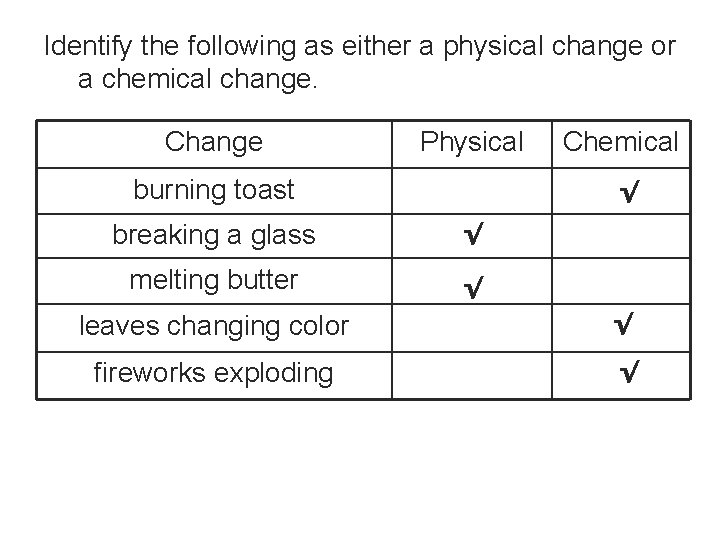
Identify the following as either a physical change or a chemical change. Change Physical burning toast Chemical √ breaking a glass √ melting butter √ leaves changing color √ fireworks exploding √

Intensive vs. Extensive n Intensive properties are properties unique to a pure substance. It is a property that can be used to identify it. Ex: Density, boiling point, melting point, odor n Extensive properties are properties that many kinds of substances can have and will not identify the substance. Ex: Mass, volume, shape

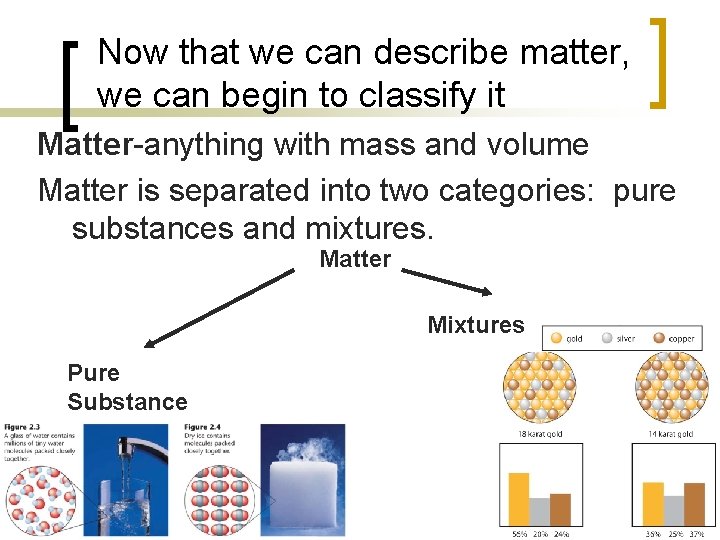
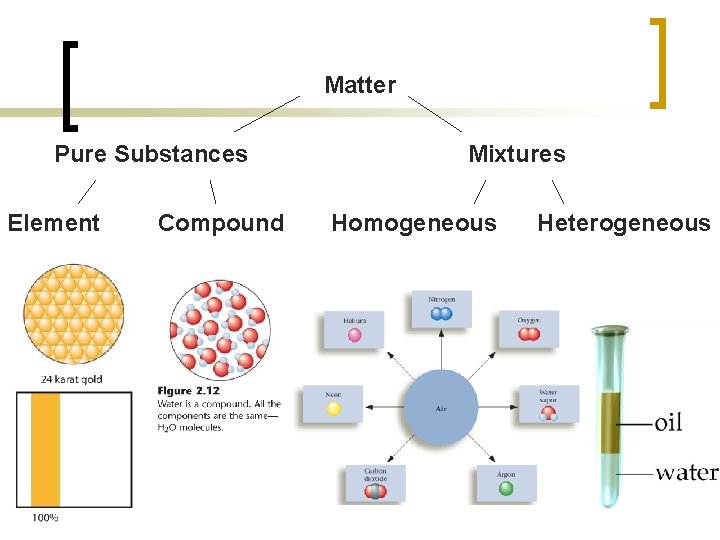
Now that we can describe matter, we can begin to classify it Matter-anything with mass and volume Matter is separated into two categories: pure substances and mixtures. Matter Mixtures Pure Substance

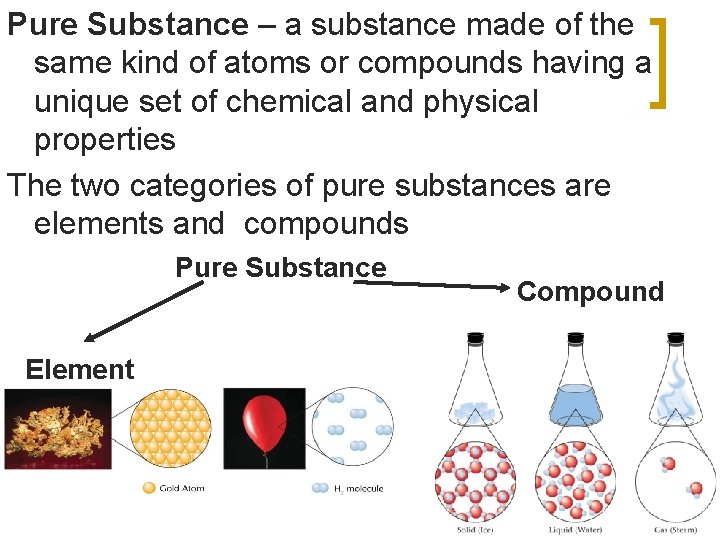
Pure Substance – a substance made of the same kind of atoms or compounds having a unique set of chemical and physical properties The two categories of pure substances are elements and compounds Pure Substance Element Compound

Element n n simplest type of pure substance made of only 1 kind of atom Examples: Hydrogen (H 2) Diatomic molecules are still Oxygen (O 2) elements. H. Br. ONCl. IF Carbon (C) all particles are identical cannot be separated by a physical or a chemical change

Compounds n made of two or more different kinds of elements chemically combined together in a specific ratio Examples: H 2 O - Water H 2 O 2 - Hydrogen Peroxide NH 3 - Ammonia n elements in a compound can only be broken apart by a chemical change

Try to sketch what a molecular view of atoms and compounds may look like Element Compound

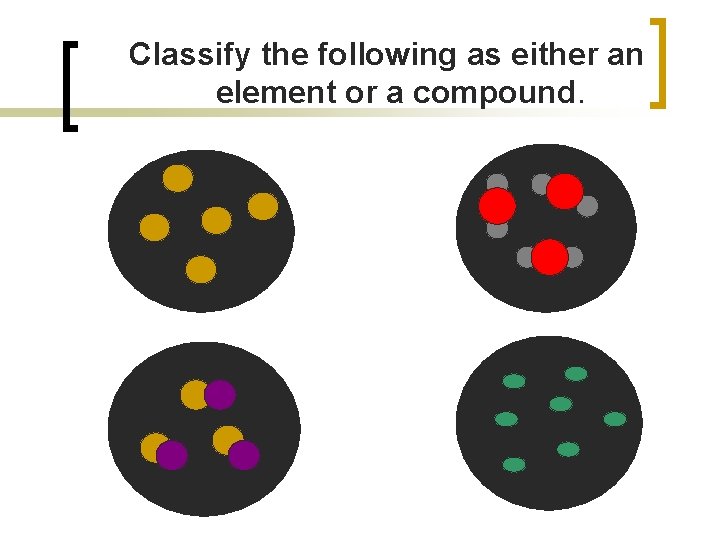
Classify the following as either an element or a compound.

Mixtures physical blend of two or more pure substances. n can be separated by a physical change The two categories of mixtures are homogeneous and heterogeneous. n Mixtures Homogeneous Mixtures Heterogeneous Mixtures

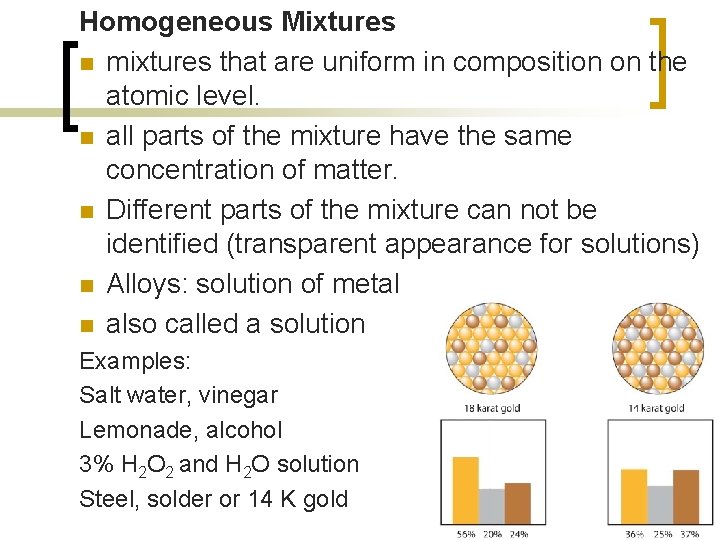
Homogeneous Mixtures n mixtures that are uniform in composition on the atomic level. n all parts of the mixture have the same concentration of matter. n Different parts of the mixture can not be identified (transparent appearance for solutions) n Alloys: solution of metal n also called a solution Examples: Salt water, vinegar Lemonade, alcohol 3% H 2 O 2 and H 2 O solution Steel, solder or 14 K gold

Heterogeneous Mixtures n mixtures that are not uniform in composition n all parts of the mixture are not the same n the different parts can usually be easily identified from one another Examples: rocks and salt and pepper oil and water Pizza

Tyndall Effect n If you shine a flashlight through a jar of a translucent colloid, the particles scatter the light, making the beam visible. Ex. Fog is an example of a colloid. This is why you can see headlights on a foggy day. 17

Ø You can use the Tyndall effect to see if your mixture is heterogeneous or homogeneous Ø If you can see the beam its heterogeneous, if you can’t it is a true solution or homogeneous 18

Try to sketch what a molecular view of atoms and compounds may look like Homogeneous Mixture Heterogeneous Mixture

Classify the following as either a homogeneous mixture or a heterogeneous mixture.

Classify the following everyday common objects as a homogeneous mixture or a heterogeneous mixture. Jelly Lotion Pizza Soda Mixed Nuts Chunky Peanut Butter

Matter Pure Substances Element Compound Mixtures Homogeneous Heterogeneous

Separating Mixtures n. Mixtures are physical blends of two or more pure substances. n. Since they are mixed physically, they can be separated into the individual pure substances by physical changes.

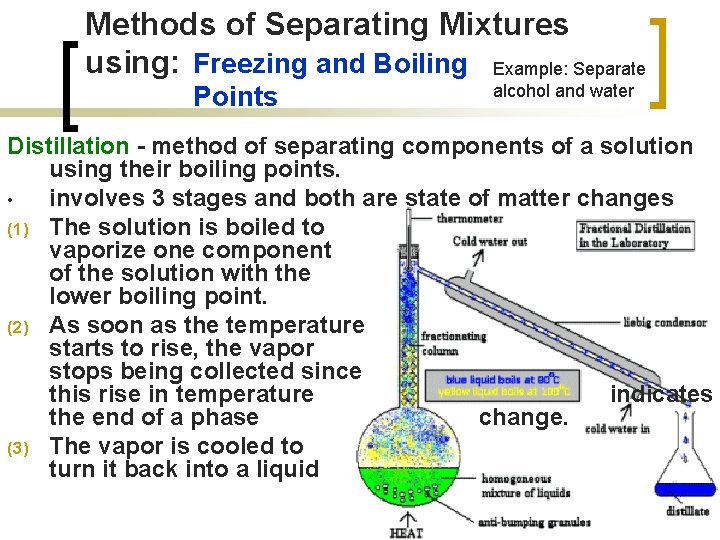
Methods of Separating Mixtures using: Freezing and Boiling Example: Separate Points alcohol and water Distillation - method of separating components of a solution using their boiling points. • involves 3 stages and both are state of matter changes (1) The solution is boiled to vaporize one component of the solution with the lower boiling point. (2) As soon as the temperature starts to rise, the vapor stops being collected since this rise in temperature indicates the end of a phase change. (3) The vapor is cooled to turn it back into a liquid

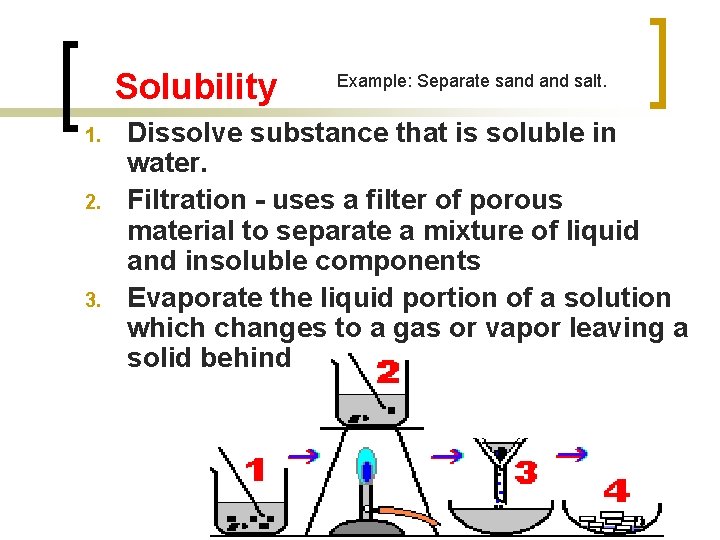
Solubility 1. 2. 3. Example: Separate sand salt. Dissolve substance that is soluble in water. Filtration - uses a filter of porous material to separate a mixture of liquid and insoluble components Evaporate the liquid portion of a solution which changes to a gas or vapor leaving a solid behind

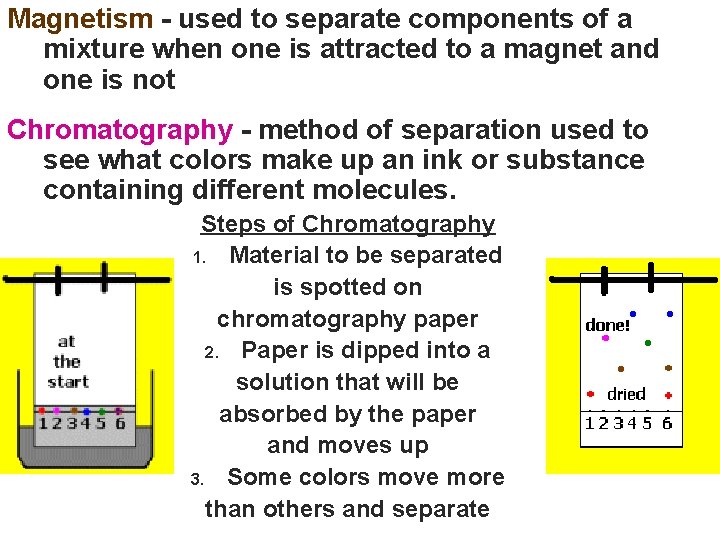
Magnetism - used to separate components of a mixture when one is attracted to a magnet and one is not Chromatography - method of separation used to see what colors make up an ink or substance containing different molecules. Steps of Chromatography 1. Material to be separated is spotted on chromatography paper 2. Paper is dipped into a solution that will be absorbed by the paper and moves up 3. Some colors move more than others and separate